Molecular Orbitals - The Oakwood School
... Molecular Orbitals Sigma Bonds – In a bonding molecular orbital of hydrogen, however, the attractions between the hydrogen nuclei and the electrons are stronger than the repulsions. – The balance of all the interactions between the hydrogen atoms is thus tipped in favor of holding the atoms togethe ...
... Molecular Orbitals Sigma Bonds – In a bonding molecular orbital of hydrogen, however, the attractions between the hydrogen nuclei and the electrons are stronger than the repulsions. – The balance of all the interactions between the hydrogen atoms is thus tipped in favor of holding the atoms togethe ...
Devillez (ld2653) – Test 1 Review – Devillez – (99998)
... foil, and the resulting deflection of the particles were observed. Most of the α particles went through the sample undeflected, suggesting that much of the atom was empty space. But of the few α particles that were deflected, they were deflected at all angles, including some very wide angles! The wi ...
... foil, and the resulting deflection of the particles were observed. Most of the α particles went through the sample undeflected, suggesting that much of the atom was empty space. But of the few α particles that were deflected, they were deflected at all angles, including some very wide angles! The wi ...
664
... Nitryl chloride may be identified by its mass spectra. The characteristic mass ions are 81, 83, 46, 35, and 37. Alternatively, nitryl chloride may be identified from its physical and chemical properties (See Reactions). The wet analytical method involves treatment with an excess solution of NaOH and ...
... Nitryl chloride may be identified by its mass spectra. The characteristic mass ions are 81, 83, 46, 35, and 37. Alternatively, nitryl chloride may be identified from its physical and chemical properties (See Reactions). The wet analytical method involves treatment with an excess solution of NaOH and ...
Syllabus Cambridge International A & AS Level Chemistry Syllabus code 9701
... In some examination sessions, two versions of the Advanced Practical Skills paper will be available, identified as Paper 31 and Paper 32. In other sessions only Paper 31 will be available. Paper 31 and Paper 32 will be equivalent and each candidate will be required to take only one of them. This is ...
... In some examination sessions, two versions of the Advanced Practical Skills paper will be available, identified as Paper 31 and Paper 32. In other sessions only Paper 31 will be available. Paper 31 and Paper 32 will be equivalent and each candidate will be required to take only one of them. This is ...
Chromatographic Enrichment of Lithium Isotopes by Hydrous
... role in nuclear science and industry. The heavy isotope of lithium, 7Li could be used as a pH control agent (7LiOH) of the coolant in nuclear fission reactors. And the lithium compounds rich in 6Li will be required for the tritium breeder blanket in deuterium-tritium fusion power reactors in the fut ...
... role in nuclear science and industry. The heavy isotope of lithium, 7Li could be used as a pH control agent (7LiOH) of the coolant in nuclear fission reactors. And the lithium compounds rich in 6Li will be required for the tritium breeder blanket in deuterium-tritium fusion power reactors in the fut ...
Chemistry 3.3 teacher led revised
... Average Atomic Masses of Elements • Average atomic mass is the weighted average of the atomic masses of the naturally occurring isotopes of an element. Calculating Average Atomic Mass • The average atomic mass of an element depends on both the mass and the relative abundance of each of the element’s ...
... Average Atomic Masses of Elements • Average atomic mass is the weighted average of the atomic masses of the naturally occurring isotopes of an element. Calculating Average Atomic Mass • The average atomic mass of an element depends on both the mass and the relative abundance of each of the element’s ...
Lewis Reeve Gibbes and the Classification of the Elements
... found himself unable to do. Thus the entire decade during which the Periodic Law was gradually emerging from the work of New-lands, Lothar Meyer, and Mendeleeff was for Gibbes one of chaos and deprivation. We cannot be surprised, therefore, that of the twelve literature references cited by Gibbes in ...
... found himself unable to do. Thus the entire decade during which the Periodic Law was gradually emerging from the work of New-lands, Lothar Meyer, and Mendeleeff was for Gibbes one of chaos and deprivation. We cannot be surprised, therefore, that of the twelve literature references cited by Gibbes in ...
chemistry - Ethiopian Ministry of Education
... 1.1.2 Major Fields of Chemistry The universe is just like a very big chemical laboratory, rearranging atoms and subatomic particles to produce elements and compounds. While planets are made up of rocks which are nothing but arrangement of compounds, an atmosphere is a mixture of compounds separated ...
... 1.1.2 Major Fields of Chemistry The universe is just like a very big chemical laboratory, rearranging atoms and subatomic particles to produce elements and compounds. While planets are made up of rocks which are nothing but arrangement of compounds, an atmosphere is a mixture of compounds separated ...
Chemistry Transition Information
... Calcium atoms each lose two electrons to form calcium ions. Chlorine atoms each gain one electron to form chloride ions. This means that calcium atoms react with chlorine atoms in the ratio of one calcium atom for every two chlorine atoms. Complete the following diagram to show the electronic struct ...
... Calcium atoms each lose two electrons to form calcium ions. Chlorine atoms each gain one electron to form chloride ions. This means that calcium atoms react with chlorine atoms in the ratio of one calcium atom for every two chlorine atoms. Complete the following diagram to show the electronic struct ...
Chapter 3: Calculations with Chemical Formulas
... consumed but before all the Al is consumed. The limiting reactant is therefore HCl. The amount of AlCl3 produced must be 0.1166, or 0.12 mol. ...
... consumed but before all the Al is consumed. The limiting reactant is therefore HCl. The amount of AlCl3 produced must be 0.1166, or 0.12 mol. ...
PRACTICE EXAM 1-C
... Potassium levels in the blood are measured in milliequivalents (mEq) of potassium per liter of blood, or “mEq/L”. A normal-range potassium level is around 4.3 mEq/L. Determine the mass of potassium contained in 1.00 pint of blood with a potassium level of 4.3 mEq/L. (7 pts) ...
... Potassium levels in the blood are measured in milliequivalents (mEq) of potassium per liter of blood, or “mEq/L”. A normal-range potassium level is around 4.3 mEq/L. Determine the mass of potassium contained in 1.00 pint of blood with a potassium level of 4.3 mEq/L. (7 pts) ...
7. A timeline of symbols and signs in chemistry
... The first interpretation is legitimate but not what was intended. The second one is not a legitimate interpretation since there is experimental evidence that the water molecule is not linear. What is likely is that the interpretation of the creator and the receiver are more likely to be the same if ...
... The first interpretation is legitimate but not what was intended. The second one is not a legitimate interpretation since there is experimental evidence that the water molecule is not linear. What is likely is that the interpretation of the creator and the receiver are more likely to be the same if ...
Gr. 11 Chemistry Student Workbook (Spring 2016)
... The most basic piece of personal protective equipment is a pair of goggles, and these will always be made available to students. Like a calculator for mathematics, and running shoes for physical education goggles are personal pieces of equipment best owned by students. When students own their own go ...
... The most basic piece of personal protective equipment is a pair of goggles, and these will always be made available to students. Like a calculator for mathematics, and running shoes for physical education goggles are personal pieces of equipment best owned by students. When students own their own go ...
Unit 1 Ch. 2,3,4 notes NEW
... and neutrons in an atom isotope - atoms which have the same number of protons and electrons but different numbers of neutrons 2:29 AM ...
... and neutrons in an atom isotope - atoms which have the same number of protons and electrons but different numbers of neutrons 2:29 AM ...
5.2 Calculations of Enthalpy Changes (SL/HL)
... There are often energy changes that take place during a chemical reaction. These may be in the form of light, sound, but much more commonly heat energy. Reactions that release heat energy are called exothermic reactions. These cause a rise in temperature. When heat energy is taken in from th ...
... There are often energy changes that take place during a chemical reaction. These may be in the form of light, sound, but much more commonly heat energy. Reactions that release heat energy are called exothermic reactions. These cause a rise in temperature. When heat energy is taken in from th ...
Stoichiometry: Calculations with Chemical Formulas and
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
Get a clicker please
... A. 50 protons, 50 electrons, 75 neutrons B . 70 neutrons, 75 protons, 50 electrons C. 120 neutrons, 50 protons, 75 electrons D. 75 electrons, 50 protons, 50 neutrons ...
... A. 50 protons, 50 electrons, 75 neutrons B . 70 neutrons, 75 protons, 50 electrons C. 120 neutrons, 50 protons, 75 electrons D. 75 electrons, 50 protons, 50 neutrons ...
Mass Relationships in Chemical Reactions
... Determining Empirical Formulas When the element % composition is known: 1. Assume 100g sample and change element percents to grams 2. Convert each to moles by dividing by molar mass of each atom ...
... Determining Empirical Formulas When the element % composition is known: 1. Assume 100g sample and change element percents to grams 2. Convert each to moles by dividing by molar mass of each atom ...
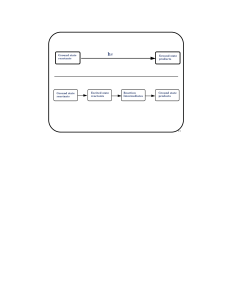
Ground state reactants Ground state products Ground state
... • It cannot occur if the sensitizer energy is significantly below 22 kcal/mol. • It can only populate the 1∆g level of molecular oxygen if the sensitizer energy is between 22 and 37 kcal/mol, since population of the 1Σg level would be energetically unfavorable. • If the sensitizer energy exceeds 38 ...
... • It cannot occur if the sensitizer energy is significantly below 22 kcal/mol. • It can only populate the 1∆g level of molecular oxygen if the sensitizer energy is between 22 and 37 kcal/mol, since population of the 1Σg level would be energetically unfavorable. • If the sensitizer energy exceeds 38 ...
Pirogov National Medical Univercity of Vinnitsa
... 2. Duty student receives the necessary work for the group equipment and reagents, and places them in the workplace. 3. In the chemical laboratory, it is permitted to work only when there is a white robe and cap. Each student is given a permanent job, which he should keep clean, do not clutter up his ...
... 2. Duty student receives the necessary work for the group equipment and reagents, and places them in the workplace. 3. In the chemical laboratory, it is permitted to work only when there is a white robe and cap. Each student is given a permanent job, which he should keep clean, do not clutter up his ...
8 Chemical Equations Chapter Outline Chemical Equations
... Write the balanced chemical equation for the reaction of magnesium hydroxide and phosphoric acid to form magnesium phosphate and water. a. 3 Mg(OH)2 + 2 H3PO4 b. Mg(OH)2 + H3PO4 ...
... Write the balanced chemical equation for the reaction of magnesium hydroxide and phosphoric acid to form magnesium phosphate and water. a. 3 Mg(OH)2 + 2 H3PO4 b. Mg(OH)2 + H3PO4 ...
Nordonia Hills City Schools Honors Chemistry Course of Study
... modern Atomic Theory from Bohr through Quantum Mechanics. 3. Relate energy levels, the s, p, d, and f sublevels (orbitals), their shapes, and their relative energies to each other. 4. Compare and contrast the contributions of key scientists (Heisenberg, Bohr, Schrodinger, Einstein, and Pauli). 5. Ex ...
... modern Atomic Theory from Bohr through Quantum Mechanics. 3. Relate energy levels, the s, p, d, and f sublevels (orbitals), their shapes, and their relative energies to each other. 4. Compare and contrast the contributions of key scientists (Heisenberg, Bohr, Schrodinger, Einstein, and Pauli). 5. Ex ...
Chapter 4
... 3. Which of the following statements best describes the charges of subatomic particles? A. Electrons have a negative charge, protons have a positive charge, and neutrons have no charge. B. Electrons have a positive charge, protons have a negative charge, and neutrons have a positive charge. C. Elect ...
... 3. Which of the following statements best describes the charges of subatomic particles? A. Electrons have a negative charge, protons have a positive charge, and neutrons have no charge. B. Electrons have a positive charge, protons have a negative charge, and neutrons have a positive charge. C. Elect ...