Subject Area Standard Area Organizing Category Grade Level
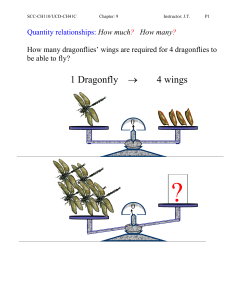
... CHEM.B.2.2.1: Utilize mathematical relationships to predict changes in the number of particles, the temperature, the pressure, and the volume in a gaseous system (i.e., Boyle’s law, Charles’s law, Dalton’s law of partial pressures, the combined gas law, and the ideal gas law). ...
... CHEM.B.2.2.1: Utilize mathematical relationships to predict changes in the number of particles, the temperature, the pressure, and the volume in a gaseous system (i.e., Boyle’s law, Charles’s law, Dalton’s law of partial pressures, the combined gas law, and the ideal gas law). ...
ism ismismismismismrapidrevisionquestionsismismismismismism
... (iii) When silicon is doped with group 15 impurities, they use four out of five electrons for covalent bond formation while the fifth electron is extra and conducts electricity. These are called n-type semiconductors. When silicon is doped with group 13 impurities, they form three covalent bonds and ...
... (iii) When silicon is doped with group 15 impurities, they use four out of five electrons for covalent bond formation while the fifth electron is extra and conducts electricity. These are called n-type semiconductors. When silicon is doped with group 13 impurities, they form three covalent bonds and ...
- Catalyst
... Possible reaction products are KCl and NH4NO3, or NH4Cl and KNO3. All are soluble, so there is no precipitate. KCl(aq) + NH4NO3 (aq) = No Reaction! Example: If a solution containing sodium sulfate is added to a solution containing barium nitrate, will a precipitate form? ...
... Possible reaction products are KCl and NH4NO3, or NH4Cl and KNO3. All are soluble, so there is no precipitate. KCl(aq) + NH4NO3 (aq) = No Reaction! Example: If a solution containing sodium sulfate is added to a solution containing barium nitrate, will a precipitate form? ...
classical damping constant
... Approaches to Collisional Broadening • Statistical effects of many particles (pressure broadening) – Usually applies to the wings, less important in the core • Some lines can be described fully by one or the other ...
... Approaches to Collisional Broadening • Statistical effects of many particles (pressure broadening) – Usually applies to the wings, less important in the core • Some lines can be described fully by one or the other ...
Production of materials
... energy to excite electrons to higher energy levels but insufficient energy to ionise atoms ...
... energy to excite electrons to higher energy levels but insufficient energy to ionise atoms ...
Stoichiometry of Chemical Reactions
... Precipitation Reactions and Solubility Rules A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displace ...
... Precipitation Reactions and Solubility Rules A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displace ...
Stoichiometry of Chemical Reactions
... Precipitation Reactions and Solubility Rules A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displace ...
... Precipitation Reactions and Solubility Rules A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displace ...
chapter:1 - WordPress.com
... The Greek concept of four elements existed for more than two thousand years. Dalton’s Atomic Theory In the beginning of 19th century Dalton proposed an atomic theory. According to this theory matter is made up of small indivisible particles called atoms. The atoms of any one element are identical in ...
... The Greek concept of four elements existed for more than two thousand years. Dalton’s Atomic Theory In the beginning of 19th century Dalton proposed an atomic theory. According to this theory matter is made up of small indivisible particles called atoms. The atoms of any one element are identical in ...
Chapter 03 - KFUPM Faculty List
... If not, when you have numbers around .5, then multiply all numbers by 2, or when you have numbers around .33 or .66, then multiply all numbers by 3. The next step is to round the numbers to the nearest integers, which gives the empirical formula: ...
... If not, when you have numbers around .5, then multiply all numbers by 2, or when you have numbers around .33 or .66, then multiply all numbers by 3. The next step is to round the numbers to the nearest integers, which gives the empirical formula: ...
Objective: To state the assumptions of Dalton`s Atomic Theory
... Millikan, and Rutherford to our understanding of the atom. ...
... Millikan, and Rutherford to our understanding of the atom. ...
Physics, Chemistry
... This paper consists of two sections. Section A will carry 45 marks and will contain a number of compulsory structured questions of variable mark value. Section B will carry 20 marks and will contain three questions, each of 10 marks. Candidates are required to answer any two questions. The questions ...
... This paper consists of two sections. Section A will carry 45 marks and will contain a number of compulsory structured questions of variable mark value. Section B will carry 20 marks and will contain three questions, each of 10 marks. Candidates are required to answer any two questions. The questions ...
1. What is the best definition of rate of reaction? A. The time it takes
... Construct the enthalpy level diagram and label the activation energy, Ea, the enthalpy change, ∆H, and the position of the transition state. ...
... Construct the enthalpy level diagram and label the activation energy, Ea, the enthalpy change, ∆H, and the position of the transition state. ...
atoms and molecules - Mockiesgateacademy
... of oxygen (O2). In accordance with the number of atoms present in these molecules, they are classified as monoatomic, diatomic, triatomic or polyatomic molecules showing that they contain one, two, three or more than three atoms respectively. ...
... of oxygen (O2). In accordance with the number of atoms present in these molecules, they are classified as monoatomic, diatomic, triatomic or polyatomic molecules showing that they contain one, two, three or more than three atoms respectively. ...
CfE Higher Chemistry Unit 1: Chemical Changes and Structure
... Collisions and concentration Look at the illustrations showing the result of collisions between two different concentrations of hydrochloric acid and calcium carbonate, both after 10 seconds of reaction. The hydrochloric acid is represented as a large sphere and the calcium carbonate as a small sphe ...
... Collisions and concentration Look at the illustrations showing the result of collisions between two different concentrations of hydrochloric acid and calcium carbonate, both after 10 seconds of reaction. The hydrochloric acid is represented as a large sphere and the calcium carbonate as a small sphe ...
Magnesium based ternary metal hydrides containing alkali and
... K. Yvon, B. Bertheville / Journal of Alloys and Compounds xxx (2006) xxx–xxx ...
... K. Yvon, B. Bertheville / Journal of Alloys and Compounds xxx (2006) xxx–xxx ...
Topological Analysis of Electron Density
... additive, but do not look very much like the balls and spheres of molecular models !!! The simple binary hydrides of the second period elements show that the relative volumes of space associated with each element is determined by their relative electronegativities. Surfaces are truncated at 0.001 au ...
... additive, but do not look very much like the balls and spheres of molecular models !!! The simple binary hydrides of the second period elements show that the relative volumes of space associated with each element is determined by their relative electronegativities. Surfaces are truncated at 0.001 au ...
Chemistry 11 Final Examination Review
... b) metallic d) compounds of polyatomic ions 26. The most active __ have the highest electronegativities. a) nonmetals b) metalloids c) metals d) noble gases 27. __ compounds have high melting points, conduct electricity in the molten phase, and tend to be soluble in water. a) hydrogen b) metallic c) ...
... b) metallic d) compounds of polyatomic ions 26. The most active __ have the highest electronegativities. a) nonmetals b) metalloids c) metals d) noble gases 27. __ compounds have high melting points, conduct electricity in the molten phase, and tend to be soluble in water. a) hydrogen b) metallic c) ...
jyvaskla2 - School of Chemistry
... additive, but do not look very much like the balls and spheres of molecular models !!! The simple binary hydrides of the second period elements show that the relative volumes of space associated with each element is determined by their relative electronegativities. Surfaces are truncated at 0.001 au ...
... additive, but do not look very much like the balls and spheres of molecular models !!! The simple binary hydrides of the second period elements show that the relative volumes of space associated with each element is determined by their relative electronegativities. Surfaces are truncated at 0.001 au ...