Chem 110 2014 - University of KwaZulu
... Brown, LeMay, Bursten, Murphy, Langford, Sagatys: Chemistry 2e © 2010 Pearson Australia ...
... Brown, LeMay, Bursten, Murphy, Langford, Sagatys: Chemistry 2e © 2010 Pearson Australia ...
Physical Chemistry II
... A solution is a homogeneous mixture of two or more substances. The substances may be in the gaseous, liquid or solid state. A homogeneous mixture is a physical mixture of two or more pure substances whose distribution is uniform throughout. When a solution forms the molecules of the solute are discr ...
... A solution is a homogeneous mixture of two or more substances. The substances may be in the gaseous, liquid or solid state. A homogeneous mixture is a physical mixture of two or more pure substances whose distribution is uniform throughout. When a solution forms the molecules of the solute are discr ...
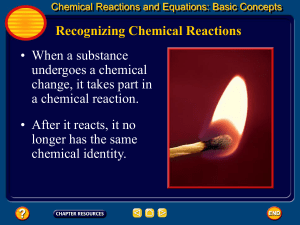
Chemical Equations
... These kinds of reactions often require a more methodical approach to balancing. In aqueous solution, these can also be balanced in acidic solution or basic solution. They are part of the general topic of oxidation and reduction, oxidation numbers, half-reactions, and electrochemistry which we won't ...
... These kinds of reactions often require a more methodical approach to balancing. In aqueous solution, these can also be balanced in acidic solution or basic solution. They are part of the general topic of oxidation and reduction, oxidation numbers, half-reactions, and electrochemistry which we won't ...
Multiple Choice
... and 5 pairs of nonbonding electrons. The 2p electron is in an excited state, otherwise it would go into 2s. The 2 2s electrons and 3 2p electrons are valence (highest energy level) five. First ionized electron is from 1s. It takes the most energy to remove electrons that are close to nucleus. The ...
... and 5 pairs of nonbonding electrons. The 2p electron is in an excited state, otherwise it would go into 2s. The 2 2s electrons and 3 2p electrons are valence (highest energy level) five. First ionized electron is from 1s. It takes the most energy to remove electrons that are close to nucleus. The ...
Chemistry 211
... •How much product is formed from some given amount of reactant? (Theoretical Yield) •How much of one reactant is required to bring a reaction to completion given some amount of another reactant? (Limiting Reagent) ...
... •How much product is formed from some given amount of reactant? (Theoretical Yield) •How much of one reactant is required to bring a reaction to completion given some amount of another reactant? (Limiting Reagent) ...
2 CHEMICAL ARITHMATICS W MODULE - 1
... different elements in a molecule of a compound. There is another kind of formula, the empirical formul of a compound, which gives only relative number of atoms of different elements. These numbers are expressed as the simplest ratio. For example, empirical formula of glucose, which consists of carbo ...
... different elements in a molecule of a compound. There is another kind of formula, the empirical formul of a compound, which gives only relative number of atoms of different elements. These numbers are expressed as the simplest ratio. For example, empirical formula of glucose, which consists of carbo ...
Subject Materials for Chemistry
... oxides some of these oxides escape into atmosphere and rest forms slag. This slag is skimmed off. When all the impurities are removed, required amount of carbon is added to molten iron to form steel of our choice. The following reactions take place in Bessemer converter. 2Mn + O2 ...
... oxides some of these oxides escape into atmosphere and rest forms slag. This slag is skimmed off. When all the impurities are removed, required amount of carbon is added to molten iron to form steel of our choice. The following reactions take place in Bessemer converter. 2Mn + O2 ...
Chemical Equations - Salem Community Schools
... Balancing an Equation Is the equation balanced now? Two sodium atoms are on each side. How many oxygen atoms are on each side? You should be able to find four on each side. How about hydrogen atoms? Now two are on each side. Because one carbon atom is still on each side, the entire equation is balan ...
... Balancing an Equation Is the equation balanced now? Two sodium atoms are on each side. How many oxygen atoms are on each side? You should be able to find four on each side. How about hydrogen atoms? Now two are on each side. Because one carbon atom is still on each side, the entire equation is balan ...
57 estonian national chemistry olympiad
... presence of coal at 1800°C, a hexagonal polymeric compound I is produced and a by-product is again gas F. Compound I is used for the manufacture of temperature-resistant ceramic products and contains equal amounts of two elements. ...
... presence of coal at 1800°C, a hexagonal polymeric compound I is produced and a by-product is again gas F. Compound I is used for the manufacture of temperature-resistant ceramic products and contains equal amounts of two elements. ...
Document
... theoretical yield: the maximum amount of product that can be formed – calculated by stoichiometry (using LR only) 1 mol Al 3 mol Cu 0.030 g Al x x = 0.0017 mol Cu 26.98 g Al 2 mol Al • This is different from the actual yield, the amount one actually produces and measures (or experimental) ...
... theoretical yield: the maximum amount of product that can be formed – calculated by stoichiometry (using LR only) 1 mol Al 3 mol Cu 0.030 g Al x x = 0.0017 mol Cu 26.98 g Al 2 mol Al • This is different from the actual yield, the amount one actually produces and measures (or experimental) ...
The Mole
... LIMITING REACTANT. The other reactant(s) are in EXCESS. To find the amount of excess, subtract the amount used from the given amount. If you have to find more than one product, be sure to start with the limiting reactant. You don’t have to determine which is the LR over and over again! ...
... LIMITING REACTANT. The other reactant(s) are in EXCESS. To find the amount of excess, subtract the amount used from the given amount. If you have to find more than one product, be sure to start with the limiting reactant. You don’t have to determine which is the LR over and over again! ...
NAME UNIT 5: BASIC ATOMIC STRUCTURE Depending upon your
... Click on this link http://www.aip.org/history/electron/jjsound.htm (or http://tinyurl.com/5t9vdfo and you can select an audiophile format that will allow you to hear JJ Thomson's voice describing the electron, from a 1934 movie, made by the J. Arthur Rank Corporation. §1.2 Early Atomic Models and ...
... Click on this link http://www.aip.org/history/electron/jjsound.htm (or http://tinyurl.com/5t9vdfo and you can select an audiophile format that will allow you to hear JJ Thomson's voice describing the electron, from a 1934 movie, made by the J. Arthur Rank Corporation. §1.2 Early Atomic Models and ...
08272012BC Science Chem 12 Chapter 1 Answer Key
... significant than the pressure as we are very close to sea level. This means molar volume is likely a bit smaller than 24.5 L/mol (or 26.5 L/mol for trial two). Dividing by a smaller number would lead to a LARGER MASS of zinc expected. 1.2 Review Questions (page 25) 1. Factors affecting heterogeneous ...
... significant than the pressure as we are very close to sea level. This means molar volume is likely a bit smaller than 24.5 L/mol (or 26.5 L/mol for trial two). Dividing by a smaller number would lead to a LARGER MASS of zinc expected. 1.2 Review Questions (page 25) 1. Factors affecting heterogeneous ...
Ceramics for catalysis
... Once the reactant is bound to the surface, it can readily undergo reactions which take place only with difficulty in the gas or liquid phases. This may result from the close proximity of reactant molecules on the surface and/or the changes in bonding consequent upon chemisorption; both are essential ...
... Once the reactant is bound to the surface, it can readily undergo reactions which take place only with difficulty in the gas or liquid phases. This may result from the close proximity of reactant molecules on the surface and/or the changes in bonding consequent upon chemisorption; both are essential ...
Chapter 2 Matter and Components F11 110
... 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass number. A sample of the element is treated as though its atoms have an average mass. ...
... 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass number. A sample of the element is treated as though its atoms have an average mass. ...
Chapter 2 Matter and Components F11 110pt
... The Modern Reassessment of the Atomic Theory 1. All matter is composed of atoms. The atom is the smallest body that retains the unique identity of the element. 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other ...
... The Modern Reassessment of the Atomic Theory 1. All matter is composed of atoms. The atom is the smallest body that retains the unique identity of the element. 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other ...
Disproportionation of Gold(II)
... fide Au2+ monometallic complexes, these results imply that solvent choice will play an important role in synthetic efforts. c. Effect of Relativity on Disproportionation. Relativistic effects are known to play a substantial role in the chemistry of gold.37 Schwerdtfeger and co-workers have compared ...
... fide Au2+ monometallic complexes, these results imply that solvent choice will play an important role in synthetic efforts. c. Effect of Relativity on Disproportionation. Relativistic effects are known to play a substantial role in the chemistry of gold.37 Schwerdtfeger and co-workers have compared ...
FREE Sample Here
... 16. Calcium chloride, CaCl2, is an ionic compound in which A. one chlorine atom transferred an electron to the other chlorine atom. B. each chlorine atom has lost electrons. C. calcium has two extra electrons in its innermost shell. D. calcium has gained two electrons. E. calcium has lost two electr ...
... 16. Calcium chloride, CaCl2, is an ionic compound in which A. one chlorine atom transferred an electron to the other chlorine atom. B. each chlorine atom has lost electrons. C. calcium has two extra electrons in its innermost shell. D. calcium has gained two electrons. E. calcium has lost two electr ...
Chapter 3 HWsolutions (from Handout)
... Step 1: Assume you have exactly 100 g of substance. 100 g is a convenient amount, because all the percentages sum to 100%. In 100 g of MSG there will be 35.51 g C, 4.77 g H, 37.85 g O, 8.29 g N, and 13.60 g Na. Step 2: Calculate the number of moles of each element in the compound. Remember, an empir ...
... Step 1: Assume you have exactly 100 g of substance. 100 g is a convenient amount, because all the percentages sum to 100%. In 100 g of MSG there will be 35.51 g C, 4.77 g H, 37.85 g O, 8.29 g N, and 13.60 g Na. Step 2: Calculate the number of moles of each element in the compound. Remember, an empir ...
ap 2005 chemistry_b scoring guidelines - AP Central
... educational organizations. Each year, the College Board serves over three and a half million students and their parents, 23,000 high schools, and 3,500 colleges through major programs and services in college admissions, guidance, assessment, financial aid, enrollment, and teaching and learning. Amon ...
... educational organizations. Each year, the College Board serves over three and a half million students and their parents, 23,000 high schools, and 3,500 colleges through major programs and services in college admissions, guidance, assessment, financial aid, enrollment, and teaching and learning. Amon ...
Chemical Bonding
... a substance that you have classified as ionic, as it is an electrolyte. Like some other ionic compounds that you are familiar with, for example, baking soda (sodium hydrogen carbonate) and chalk (calcium carbonate), it is also brittle and has a high melting temperature. How do we explain the formati ...
... a substance that you have classified as ionic, as it is an electrolyte. Like some other ionic compounds that you are familiar with, for example, baking soda (sodium hydrogen carbonate) and chalk (calcium carbonate), it is also brittle and has a high melting temperature. How do we explain the formati ...
Chemistry XXI
... Go back and analyze the notes for the decomposition of Alanine. Based on our overall results, analyze the likelihood of amino acids forming in hydrothermal vents on the primitive Earth. The strong dependence on T of the decomposition of amino acids makes it difficult to decide whether the “hydrother ...
... Go back and analyze the notes for the decomposition of Alanine. Based on our overall results, analyze the likelihood of amino acids forming in hydrothermal vents on the primitive Earth. The strong dependence on T of the decomposition of amino acids makes it difficult to decide whether the “hydrother ...
Atomic Masses
... C(s) + O2 (g) CO2 (g) of carbon 1 Atom reacts with 1 Molecule to yield 1 Molecule • But individual atoms are to small to see • We can easily count things like jelly beans and pennies, but atoms are far too small to be counted ...
... C(s) + O2 (g) CO2 (g) of carbon 1 Atom reacts with 1 Molecule to yield 1 Molecule • But individual atoms are to small to see • We can easily count things like jelly beans and pennies, but atoms are far too small to be counted ...
Chapter 3: Elements, Compounds and the Periodic Table
... Metals, Nonmetals, or Metalloids Elements break down into 3 broad categories Organized by regions of periodic table Metals Left-hand side Sodium, lead, iron, gold ...
... Metals, Nonmetals, or Metalloids Elements break down into 3 broad categories Organized by regions of periodic table Metals Left-hand side Sodium, lead, iron, gold ...