SELECTED ANSWERS
... average distance from other particles and in the same general position with respect to its neighbors. (b) The velocity of the particles increases, causing more violent collisions between them. This causes them to move apart, so the solid expands. See Figure 2.1. (c) The particles break out of their ...
... average distance from other particles and in the same general position with respect to its neighbors. (b) The velocity of the particles increases, causing more violent collisions between them. This causes them to move apart, so the solid expands. See Figure 2.1. (c) The particles break out of their ...
ch03 - Atoms and Elements
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
11.1 Enthalpy PowerPoint
... consists of an insulated container made of three nested polystyrene cups, a measured quantity of water, and a thermometer. The chemical is placed in or dissolved in the water of the calorimeter. Energy transfers between the chemical system and the surrounding water is monitored by measuring changes ...
... consists of an insulated container made of three nested polystyrene cups, a measured quantity of water, and a thermometer. The chemical is placed in or dissolved in the water of the calorimeter. Energy transfers between the chemical system and the surrounding water is monitored by measuring changes ...
Chapter 10: Nuclear Physics
... • We know that radioactive nuclei can spontaneously change into nuclei of other elements, a process called transmutation. • Scientists wondered if the reverse was possible. • Could a particle (proton or neutron) be added to a nucleus to change it into another element? • The answer is “yes,” and this ...
... • We know that radioactive nuclei can spontaneously change into nuclei of other elements, a process called transmutation. • Scientists wondered if the reverse was possible. • Could a particle (proton or neutron) be added to a nucleus to change it into another element? • The answer is “yes,” and this ...
POGIL - Basic Skills Supplement - The Mole-1
... A mole is a way to quantify atoms and molecules. The mole is a critical component when working with chemical reactions. The purpose of the mole is to normalize quantities of atoms and molecules when working with them in chemical reactions. In a molecule of water (H2O), for example, there are two hy ...
... A mole is a way to quantify atoms and molecules. The mole is a critical component when working with chemical reactions. The purpose of the mole is to normalize quantities of atoms and molecules when working with them in chemical reactions. In a molecule of water (H2O), for example, there are two hy ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... • Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. 4. Fluorine always has an oxidation number of −1. 5. The other halogens have an oxidation number of −1 when they are negative (they can have positive oxidation numbers, however, most notably ...
... • Nonmetals tend to have negative oxidation numbers, although some are positive in certain compounds or ions. 4. Fluorine always has an oxidation number of −1. 5. The other halogens have an oxidation number of −1 when they are negative (they can have positive oxidation numbers, however, most notably ...
C - Thierry Karsenti
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
Chapter 1 – Reaction Kinetics Answer Key
... during a chemical reaction. The amount (number of moles) certainly does change. However, we must realize that as more moles of solid or liquid are formed, the volume of the solid or liquid al ...
... during a chemical reaction. The amount (number of moles) certainly does change. However, we must realize that as more moles of solid or liquid are formed, the volume of the solid or liquid al ...
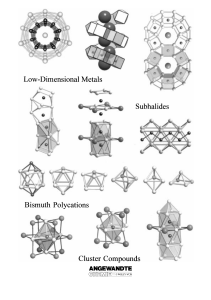
From the Metal to the Molecule
... Such subvalent compounds are not only known for electron-deficient s-, d-, and f-metals, but also for p-(semi-) metals albeit with far fewer examples. For example, recently significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research m ...
... Such subvalent compounds are not only known for electron-deficient s-, d-, and f-metals, but also for p-(semi-) metals albeit with far fewer examples. For example, recently significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research m ...
thermdyn - chemmybear.com
... The first ionization energy of sodium is +496 ki= 226.3 -[4(5.69) + 4(130.6) + 205] = -523.9 J/K lojoules per mole, yet the standard heat of formation of (d) Gf = Hf - TSf = 533.8 - (298)(-0.5239) kJ sodium chloride from its elements in their standard state is -411 kilojoules per mole. = -377. ...
... The first ionization energy of sodium is +496 ki= 226.3 -[4(5.69) + 4(130.6) + 205] = -523.9 J/K lojoules per mole, yet the standard heat of formation of (d) Gf = Hf - TSf = 533.8 - (298)(-0.5239) kJ sodium chloride from its elements in their standard state is -411 kilojoules per mole. = -377. ...
Physical Chemistry 2.pdf
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
Chemical Reactions - Effingham County Schools
... A mole ratio is a conversion factor that relates the amounts in moles of any two substances involved in a chemical reaction 2Al2O3(l) → 4Al(s) + 3O2(g) 2 mol Al2O3 4 mol Al ...
... A mole ratio is a conversion factor that relates the amounts in moles of any two substances involved in a chemical reaction 2Al2O3(l) → 4Al(s) + 3O2(g) 2 mol Al2O3 4 mol Al ...
Unit 2 Summary - A
... the hydrolysis of bromoethane: C2H5Br + NaOH C2H5OH + NaBr the fermentation of glucose: C6H12O6 2C2H5OH + 2CO2 the hydration of ethane: C2H4 + H2O C2H5OH (o) describe the benefits of developing chemical processes with a high atom economy in terms of fewer waste materials; Why is it an advantag ...
... the hydrolysis of bromoethane: C2H5Br + NaOH C2H5OH + NaBr the fermentation of glucose: C6H12O6 2C2H5OH + 2CO2 the hydration of ethane: C2H4 + H2O C2H5OH (o) describe the benefits of developing chemical processes with a high atom economy in terms of fewer waste materials; Why is it an advantag ...
1 Intro / Review : Chemical Kinetics
... Enduring understanding 4.B: Elementary reactions are mediated by collisions between molecules. Only collisions having sufficient energy and proper relative orientation of reactants lead to products. Enduring understanding 4.C: Many reactions proceed via a series of elementary reactions. Essential kn ...
... Enduring understanding 4.B: Elementary reactions are mediated by collisions between molecules. Only collisions having sufficient energy and proper relative orientation of reactants lead to products. Enduring understanding 4.C: Many reactions proceed via a series of elementary reactions. Essential kn ...
Chemistry Spell check on
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. Write your answers clearly in the spaces provided in this booklet. Additional space for answers and rough work is provided at the end of this booklet. If you use this space you must clearly identify the question number y ...
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. Write your answers clearly in the spaces provided in this booklet. Additional space for answers and rough work is provided at the end of this booklet. If you use this space you must clearly identify the question number y ...
Document
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. Write your answers clearly in the spaces provided in this booklet. Additional space for answers and rough work is provided at the end of this booklet. If you use this space you must clearly identify the question number y ...
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. Write your answers clearly in the spaces provided in this booklet. Additional space for answers and rough work is provided at the end of this booklet. If you use this space you must clearly identify the question number y ...
Chemistry 8.2
... Double-Displacement Reactions • In double-displacement reactions, the ions of two compounds exchange places in an aqueous solution to form two new compounds. • One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually wa ...
... Double-Displacement Reactions • In double-displacement reactions, the ions of two compounds exchange places in an aqueous solution to form two new compounds. • One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually wa ...
PDF on arxiv.org - at www.arxiv.org.
... The “chemical bond” is a central concept in molecular sciences, but there is no consensus as to what a bond actually is. Therefore, a variety of bonding models have been developed, each defining the structure of molecules in a different manner with the goal of explaining and predicting chemical prop ...
... The “chemical bond” is a central concept in molecular sciences, but there is no consensus as to what a bond actually is. Therefore, a variety of bonding models have been developed, each defining the structure of molecules in a different manner with the goal of explaining and predicting chemical prop ...
Chem 110 2014 - University of KwaZulu
... Brown, LeMay, Bursten, Murphy, Langford, Sagatys: Chemistry 2e © 2010 Pearson Australia ...
... Brown, LeMay, Bursten, Murphy, Langford, Sagatys: Chemistry 2e © 2010 Pearson Australia ...