Chapter 4
... 3. Which of the following statements best describes the charges of subatomic particles? A. Electrons have a negative charge, protons have a positive charge, and neutrons have no charge. B. Electrons have a positive charge, protons have a negative charge, and neutrons have a positive charge. C. Elect ...
... 3. Which of the following statements best describes the charges of subatomic particles? A. Electrons have a negative charge, protons have a positive charge, and neutrons have no charge. B. Electrons have a positive charge, protons have a negative charge, and neutrons have a positive charge. C. Elect ...
Chapter 8 Concepts of Chemical Bonding
... • G. N. Lewis developed a method to denote potential bonding electrons by using one dot for every valence electron around the element symbol. • When forming compounds, atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons - electrons involved in bonding ( ...
... • G. N. Lewis developed a method to denote potential bonding electrons by using one dot for every valence electron around the element symbol. • When forming compounds, atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons - electrons involved in bonding ( ...
Compounds of Chlorine
... The structure of ClO2 (Figure 4b) is equivalent to SO2 with one extra electron, resulting in a paramagnetic unpaired electron species. Unusually, despite the unpaired electron con guration, ClO2 shows no tendency to dimerize. This is unlike the analogous NO2 molecule. Dichlorine tetraoxide (Cl2 O4 ) ...
... The structure of ClO2 (Figure 4b) is equivalent to SO2 with one extra electron, resulting in a paramagnetic unpaired electron species. Unusually, despite the unpaired electron con guration, ClO2 shows no tendency to dimerize. This is unlike the analogous NO2 molecule. Dichlorine tetraoxide (Cl2 O4 ) ...
File
... n textbook — the bombarding electrons in a mass spectrometer have a high kinetic energy n answer — the electrons are fast moving Some answers are expanded to show the logic of the calculation. For instance, the conversion of 23.7 cm3 of solution to dm3 in the answer to Question 9 of the first chap ...
... n textbook — the bombarding electrons in a mass spectrometer have a high kinetic energy n answer — the electrons are fast moving Some answers are expanded to show the logic of the calculation. For instance, the conversion of 23.7 cm3 of solution to dm3 in the answer to Question 9 of the first chap ...
Chapter -
... 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 2C2H6 ...
... 2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each element the same on both sides of the equation. Do not change the subscripts. 2C2H6 ...
Week 3 July 22, 2016 Worksheet Review III 1 mol = 6.022 × 1023 1
... 1.807 × 1024 atoms are present in the sample True. We know that for every 1 molecule of H2O, we have 2 atoms of H and 1 atom of O. So we every 1 molecule of H2O has 3 atoms. 3× (6.022 × 1023 molecules H 2O) = 1.807 × 1024 atoms ...
... 1.807 × 1024 atoms are present in the sample True. We know that for every 1 molecule of H2O, we have 2 atoms of H and 1 atom of O. So we every 1 molecule of H2O has 3 atoms. 3× (6.022 × 1023 molecules H 2O) = 1.807 × 1024 atoms ...
Ch 4 Student.pptx
... If the actual yield for the previous problem was 10.5 g, calculate the percent yield. The theoretical yield that we calculated was 13.6 g. If the actual yield is 3.16 g then percent yield is ...
... If the actual yield for the previous problem was 10.5 g, calculate the percent yield. The theoretical yield that we calculated was 13.6 g. If the actual yield is 3.16 g then percent yield is ...
Spring 2008
... There are more molecules in the gas phase on the LH side, to increasing the P drives it to the right 27. For the reaction: aA(g) + bB(g) cC(g) + heat with a = 1, b=1 and c=3. An increase in total pressure (at const T) A. increases the equilibrium constant B. decreases the equilibrium constant C. doe ...
... There are more molecules in the gas phase on the LH side, to increasing the P drives it to the right 27. For the reaction: aA(g) + bB(g) cC(g) + heat with a = 1, b=1 and c=3. An increase in total pressure (at const T) A. increases the equilibrium constant B. decreases the equilibrium constant C. doe ...
Chapter 3 Chemical Reactions and Reaction Stoichiometry
... Begin by counting each kind of atom on the two sides of the arrow. There are one Na, one O, and two H on the left side, and one Na, one O, and three H on the right. The Na and O atoms are balanced, but the number of H atoms is not. To increase the number of H atoms on the left, let’s try placing the ...
... Begin by counting each kind of atom on the two sides of the arrow. There are one Na, one O, and two H on the left side, and one Na, one O, and three H on the right. The Na and O atoms are balanced, but the number of H atoms is not. To increase the number of H atoms on the left, let’s try placing the ...
Liquid chromatography: a tool for the analysis of metal species
... (iv) multidimensional and multimode chromatography. Multidimensional and multimode chromatography involve, respectively: (i) the use of two different mechanisms (e.g. ion-exchange coupled with ionexclusion); (ii) the use of two or more columns, switching the total flux or a portion of eluate from on ...
... (iv) multidimensional and multimode chromatography. Multidimensional and multimode chromatography involve, respectively: (i) the use of two different mechanisms (e.g. ion-exchange coupled with ionexclusion); (ii) the use of two or more columns, switching the total flux or a portion of eluate from on ...
CHEM 1411 – STUDY-GUIDE-for-TEST-2
... 56. During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation: 2H2S(g) + 3O2(g) 2SO2(g) + 2H2O(g) Calculate the standard enthalpy change for the above reaction given: 3S(s) + 2H2O(g) 2H2S(g) + SO2(g) H° = 146.9 kJ/mol S(s) + O2 ...
... 56. During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation: 2H2S(g) + 3O2(g) 2SO2(g) + 2H2O(g) Calculate the standard enthalpy change for the above reaction given: 3S(s) + 2H2O(g) 2H2S(g) + SO2(g) H° = 146.9 kJ/mol S(s) + O2 ...
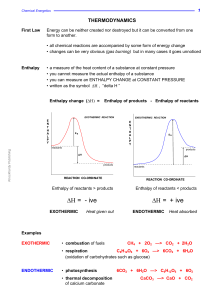
Η - Knockhardy
... 25cm3 of 2.0M HCl was added to 25cm3 of 2.0M NaOH in an insulated beaker. The initial temperature of both solutions was 20°C. The reaction mixture was stirred to ensure mixing and the highest temperature reached by the solution was 33°C. Calculate the Molar Enthalpy of Neutralisation. Temperature ri ...
... 25cm3 of 2.0M HCl was added to 25cm3 of 2.0M NaOH in an insulated beaker. The initial temperature of both solutions was 20°C. The reaction mixture was stirred to ensure mixing and the highest temperature reached by the solution was 33°C. Calculate the Molar Enthalpy of Neutralisation. Temperature ri ...
APPLICATION OF IONIC LIQUIDS IN ORGANIC SYNTHESIS
... Conventional organic solvents are used in a range of pharmaceutical and industrial applications. Ionic liquids have been gaining growing attention from synthetic organic chemists and practitioners in chemical industries in general. Ionic liquids are defined as containing organic cations and inorgani ...
... Conventional organic solvents are used in a range of pharmaceutical and industrial applications. Ionic liquids have been gaining growing attention from synthetic organic chemists and practitioners in chemical industries in general. Ionic liquids are defined as containing organic cations and inorgani ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... Adipic acid, H2C6H8O4, used to produce nylon, is made commercially by a reaction between cyclohexane (C6H12) and O2: 2 C6H12(l) + 5 O2(g) 2 H2C6H8O4(l) + 2 H2O(g) (a) Assume that you carry out this reaction with 25.0 g of cyclohexane and that cyclohexane is the limiting reactant. What is the theoret ...
... Adipic acid, H2C6H8O4, used to produce nylon, is made commercially by a reaction between cyclohexane (C6H12) and O2: 2 C6H12(l) + 5 O2(g) 2 H2C6H8O4(l) + 2 H2O(g) (a) Assume that you carry out this reaction with 25.0 g of cyclohexane and that cyclohexane is the limiting reactant. What is the theoret ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... One mole of atoms, ions, or molecules contains Avogadro’s number of those particles One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... One mole of atoms, ions, or molecules contains Avogadro’s number of those particles One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
Chemistry Essentials For Dummies
... Greenville, South Carolina. After a stint in the United States Army, he decided to try his hand at teaching. In 1971, he joined the chemistry faculty of Stephen F. Austin State University in Nacogdoches, Texas where he still teaches chemistry. In 1985, he started back to school part time and in 1991 ...
... Greenville, South Carolina. After a stint in the United States Army, he decided to try his hand at teaching. In 1971, he joined the chemistry faculty of Stephen F. Austin State University in Nacogdoches, Texas where he still teaches chemistry. In 1985, he started back to school part time and in 1991 ...
ordinary level chemistry syllabus
... I wish to sincerely extend my special appreciation to the people who played a major role in the development of this syllabus. It would not have been successful without the participation of a range of different education stakeholders and the financial support from different donors. For this I would l ...
... I wish to sincerely extend my special appreciation to the people who played a major role in the development of this syllabus. It would not have been successful without the participation of a range of different education stakeholders and the financial support from different donors. For this I would l ...
acid
... Weak acids and bases - Molecular compounds that are weak acids or weak bases are also weak electrolytes. Note that an acid forms H+ ion when added to water, and a base forms OH- ion. ...
... Weak acids and bases - Molecular compounds that are weak acids or weak bases are also weak electrolytes. Note that an acid forms H+ ion when added to water, and a base forms OH- ion. ...
Dr David`s Chemistry Test Answers
... Higher pressure and lower temperature would favour the left to right reaction. However, a higher pressure would make the process less economic due to the increased costs of equipment and maintenance and a lower temperature would slow the reaction again reducing the overall economy. 2. Nitric acid th ...
... Higher pressure and lower temperature would favour the left to right reaction. However, a higher pressure would make the process less economic due to the increased costs of equipment and maintenance and a lower temperature would slow the reaction again reducing the overall economy. 2. Nitric acid th ...
SQA CfE Higher Chemistry Unit 1: Chemical Changes and Structure
... Collision theory, based on the kinetic model of matter, provides an explanation for the effect that various factors have on the rate of chemical reactions in terms of the number of successful collisions which occur. Collision theory can be stated thus: • particles must collide to react. • not all co ...
... Collision theory, based on the kinetic model of matter, provides an explanation for the effect that various factors have on the rate of chemical reactions in terms of the number of successful collisions which occur. Collision theory can be stated thus: • particles must collide to react. • not all co ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... One mole of atoms, ions, or molecules contains Avogadro’s number of those particles One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... One mole of atoms, ions, or molecules contains Avogadro’s number of those particles One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
2014_S4_CHM_NORMAL (ALL)
... 43. The electronic structure of a compound formed between an element X and chlorine is shown below. (Only electrons in the outermost shells are shown.) What would be the formula of the compound formed between X and magnesium? A. ...
... 43. The electronic structure of a compound formed between an element X and chlorine is shown below. (Only electrons in the outermost shells are shown.) What would be the formula of the compound formed between X and magnesium? A. ...
CHEMISTRY Academic Standards Statement
... i. The mole concept is a unifying concept for describing/measuring quantities of substances. It relates the macroscale (mass) to the microscale (atoms, molecules etc.). ii. Stoichiometry is the unique numerical relationship by which atoms, ions and molecules combine together. iii. Electrons, protons ...
... i. The mole concept is a unifying concept for describing/measuring quantities of substances. It relates the macroscale (mass) to the microscale (atoms, molecules etc.). ii. Stoichiometry is the unique numerical relationship by which atoms, ions and molecules combine together. iii. Electrons, protons ...
unit iv – stoichiometry 1
... * Chemical Formulas - a series of symbols and numbers used to represent the composition of an element or a compound I. Formulas for Compounds A. Ionic Compounds - represents ratio of cations to anions * remember, ionic compounds don’t form molecules as we think of them * we call “molecules” formula ...
... * Chemical Formulas - a series of symbols and numbers used to represent the composition of an element or a compound I. Formulas for Compounds A. Ionic Compounds - represents ratio of cations to anions * remember, ionic compounds don’t form molecules as we think of them * we call “molecules” formula ...