An Efficient Oxidation of Benzoins to Benzils by Manganese (II
... An oxygen molecule from decomposition of H2 O2 reacts with Mn(II) to form an oxomanganese complex A [35, 36], which undergoes oxidative addition to benzoin and gives an intermediate � which �nally undergoes reductive elimination to give the desired product [37] and Mn(II) is regenerated back. As sho ...
... An oxygen molecule from decomposition of H2 O2 reacts with Mn(II) to form an oxomanganese complex A [35, 36], which undergoes oxidative addition to benzoin and gives an intermediate � which �nally undergoes reductive elimination to give the desired product [37] and Mn(II) is regenerated back. As sho ...
Stoichiometry - Madison Public Schools
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
Document
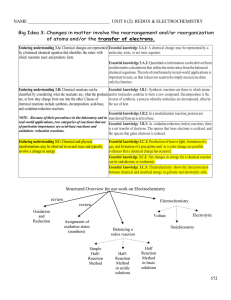
... 1.5, 7.1; Essential knowledge components of 3.A–3.C] Learning objective 3.2 The student can translate an observed chemical change into a balanced chemical equation and justify the choice of equation type (molecular, ionic, or net ionic) in terms of utility for the given circumstances. [See SP 1.5, 7 ...
... 1.5, 7.1; Essential knowledge components of 3.A–3.C] Learning objective 3.2 The student can translate an observed chemical change into a balanced chemical equation and justify the choice of equation type (molecular, ionic, or net ionic) in terms of utility for the given circumstances. [See SP 1.5, 7 ...
WRITING CHEMICAL FORMULAE
... 3) Find the concentration in mol l-1 of these solutions:In question 3 you have to find the concentration of a solution starting from a mass of solute. The first step is to use the GFM to convert the mass to moles. (Remember that number of moles = mass in grams gram formula mass) Then continue as i ...
... 3) Find the concentration in mol l-1 of these solutions:In question 3 you have to find the concentration of a solution starting from a mass of solute. The first step is to use the GFM to convert the mass to moles. (Remember that number of moles = mass in grams gram formula mass) Then continue as i ...
NUCL 1 Early life of Albert Ghiorso: Preparation for future role as
... Early life of Albert Ghiorso: Preparation for future role as innovator and alchemist Darleane Christian Hoffman, [email protected]. Division of Nuclear Science, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, United States Although young Albert was a good student in classical subjects, he ...
... Early life of Albert Ghiorso: Preparation for future role as innovator and alchemist Darleane Christian Hoffman, [email protected]. Division of Nuclear Science, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, United States Although young Albert was a good student in classical subjects, he ...
CHAPTER 16
... Exothermic compounds tend to be very stable. If a large amount of energy as heat is released when a compound is formed, the compound has a large negative enthalpy of formation. Such compounds are very stable. Elements in their standard states are defined as having ∆H f0 = 0. The ∆H f0 of carbon diox ...
... Exothermic compounds tend to be very stable. If a large amount of energy as heat is released when a compound is formed, the compound has a large negative enthalpy of formation. Such compounds are very stable. Elements in their standard states are defined as having ∆H f0 = 0. The ∆H f0 of carbon diox ...
CHEMISTRY - careerpoint.ac.in
... There are five laws which were first proposed in the 18th century and early of 19th century to NOTES be followed by the chemical reactions. At that time these laws was universally accepted by the scientists, because each of these laws was supported by the Dalton's atomic theory. After the discovery ...
... There are five laws which were first proposed in the 18th century and early of 19th century to NOTES be followed by the chemical reactions. At that time these laws was universally accepted by the scientists, because each of these laws was supported by the Dalton's atomic theory. After the discovery ...
Document
... 1.5, 7.1; Essential knowledge components of 3.A–3.C] Learning objective 3.2 The student can translate an observed chemical change into a balanced chemical equation and justify the choice of equation type (molecular, ionic, or net ionic) in terms of utility for the given circumstances. [See SP 1.5, 7 ...
... 1.5, 7.1; Essential knowledge components of 3.A–3.C] Learning objective 3.2 The student can translate an observed chemical change into a balanced chemical equation and justify the choice of equation type (molecular, ionic, or net ionic) in terms of utility for the given circumstances. [See SP 1.5, 7 ...
Thermodynamics ppt
... Thermodynamics - ch 10 Entropy (S) Considerations: 1. S(s) < S(l) << S(g) 2. There is more entropy at higher temperatures and/or larger volumes (lower pressures) 3. The more bonds per molecule the greater the positional probability ex: CH4 > H2 4. If there are the same number of atoms in the molecu ...
... Thermodynamics - ch 10 Entropy (S) Considerations: 1. S(s) < S(l) << S(g) 2. There is more entropy at higher temperatures and/or larger volumes (lower pressures) 3. The more bonds per molecule the greater the positional probability ex: CH4 > H2 4. If there are the same number of atoms in the molecu ...
Instructor`s Guide to General Chemistry: Guided
... (a) The balanced reaction equation is needed to relate the number of molecules/ions of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be ...
... (a) The balanced reaction equation is needed to relate the number of molecules/ions of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be ...
Coordination and Chemistry of Stable Cu (II) Complexes in the Gas
... molecular beam, which is then ionized by high-energy electron impact. The success of this technique relies on the fact that multiply charged metal ions are generated after the metal has already been encapsulated within a stable solvent environment, which circumvents the need for any growth mechanism ...
... molecular beam, which is then ionized by high-energy electron impact. The success of this technique relies on the fact that multiply charged metal ions are generated after the metal has already been encapsulated within a stable solvent environment, which circumvents the need for any growth mechanism ...
Atomic Target Practice
... have been different if the marble speed had been faster or slower? The most obvious answer students will give is that the marble speed must be fast enough that the marble will penetrate the “black box” completely if the target is not in its path. Note to teachers: The speed of the marble will also ...
... have been different if the marble speed had been faster or slower? The most obvious answer students will give is that the marble speed must be fast enough that the marble will penetrate the “black box” completely if the target is not in its path. Note to teachers: The speed of the marble will also ...
Scoring Guidelines - AP Central
... 5. An experiment is performed to determine the molar mass of an unknown solid monoprotic acid, HA, by titration with a standardized NaOH solution. (a) What measurement(s) must be made to determine the number of moles of NaOH used in the titration? Initial volume of standardized NaOH solution and fin ...
... 5. An experiment is performed to determine the molar mass of an unknown solid monoprotic acid, HA, by titration with a standardized NaOH solution. (a) What measurement(s) must be made to determine the number of moles of NaOH used in the titration? Initial volume of standardized NaOH solution and fin ...
AP Chemistry Curriculum Map - Belle Vernon Area School District
... orbitals with electrons, distribution of electrons in orbitals, shapes of orbitals). Anchor: CHEM.A.2.3 – Explain how periodic trends in the properties of atoms allow for the prediction of physical and chemical properties. Eligible Content CHEM.A.2.3.1 – Explain how the periodicity of chemical pro ...
... orbitals with electrons, distribution of electrons in orbitals, shapes of orbitals). Anchor: CHEM.A.2.3 – Explain how periodic trends in the properties of atoms allow for the prediction of physical and chemical properties. Eligible Content CHEM.A.2.3.1 – Explain how the periodicity of chemical pro ...
The Mole - Bakersfield College
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Dr. Spencer`s PPT
... Paris, and although Lavoisier's father wanted him to be a lawyer, Lavoisier was fascinated by science. From the beginning of his scientific career, Lavoisier recognized the importance of accurate measurements. He wrote the first modern chemistry (1789) textbook so that it is not surprising that Lavo ...
... Paris, and although Lavoisier's father wanted him to be a lawyer, Lavoisier was fascinated by science. From the beginning of his scientific career, Lavoisier recognized the importance of accurate measurements. He wrote the first modern chemistry (1789) textbook so that it is not surprising that Lavo ...
Study guide for final
... 13) When the number 65.59 is rounded to contain 2 significant figures, it becomes 66.0. 14) Conversion factors are constructed from any two quantities known to be equivalent. 15) Liquid and gas molecules can easily be compressed, while in a solid the molecules are incompressible. 16) A chemical chan ...
... 13) When the number 65.59 is rounded to contain 2 significant figures, it becomes 66.0. 14) Conversion factors are constructed from any two quantities known to be equivalent. 15) Liquid and gas molecules can easily be compressed, while in a solid the molecules are incompressible. 16) A chemical chan ...
Chemistry 30 - SharpSchool
... 30-D2.3k calculate equilibrium constants and concentrations for homogeneous systems and Bronsted-Lowry acids and bases (excluding buffers) when concentrations at equilibrium are known initial concentrations and one equilibrium concentration are known the equilibrium constant and one equilibr ...
... 30-D2.3k calculate equilibrium constants and concentrations for homogeneous systems and Bronsted-Lowry acids and bases (excluding buffers) when concentrations at equilibrium are known initial concentrations and one equilibrium concentration are known the equilibrium constant and one equilibr ...
DOE Chemistry 1
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
NOBLE-GAS CHEMISTRY
... low coordination number of a metal center, has been provided by transU(C)(O)Ng4 complexes (Ng = Ar, Kr, Xe).32,33 Another impressive piece of research came from the field of supersonic jets. In a series of landmark papers appearing since 2000, Gerry and co-workers have reported that noble-gas atoms ...
... low coordination number of a metal center, has been provided by transU(C)(O)Ng4 complexes (Ng = Ar, Kr, Xe).32,33 Another impressive piece of research came from the field of supersonic jets. In a series of landmark papers appearing since 2000, Gerry and co-workers have reported that noble-gas atoms ...
BC Science 10 Workbook Answers
... 2. PCBs were used for industrial products, such as heat exchange fluids, paints, plastics, and lubricants for electrical transformers. 3. PCBs stay in the environment for a long time. Aquatic ecosystems and species that feed on aquatic organisms are especially sensitive to the effects of PCBs. PCBs ...
... 2. PCBs were used for industrial products, such as heat exchange fluids, paints, plastics, and lubricants for electrical transformers. 3. PCBs stay in the environment for a long time. Aquatic ecosystems and species that feed on aquatic organisms are especially sensitive to the effects of PCBs. PCBs ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... convert 9.0 g of glucose into moles (MM 180) convert moles of glucose into moles of water convert moles of water into grams (MM 18.02) convert grams of water into mL a) How? what is the relationship between mass and volume? density of water = 1.00 g/mL ...
... convert 9.0 g of glucose into moles (MM 180) convert moles of glucose into moles of water convert moles of water into grams (MM 18.02) convert grams of water into mL a) How? what is the relationship between mass and volume? density of water = 1.00 g/mL ...