Chemistry - Set as Home Page
... 10 ml of 3 M HCl was titrated with a standard solution of NaOH containing 80 grams per litre. The volume of the standard solution required to neutralize the acid would be _________ ml. ...
... 10 ml of 3 M HCl was titrated with a standard solution of NaOH containing 80 grams per litre. The volume of the standard solution required to neutralize the acid would be _________ ml. ...
Preview Sample 2
... 53. Which of the following statements about Mendeleev's periodic table is incorrect? A. Mendeleev arranged the known elements in order of increasing relative atomic mass. B. He grouped elements with similar properties into columns and rows so that their properties varied in a regular pattern. C. He ...
... 53. Which of the following statements about Mendeleev's periodic table is incorrect? A. Mendeleev arranged the known elements in order of increasing relative atomic mass. B. He grouped elements with similar properties into columns and rows so that their properties varied in a regular pattern. C. He ...
2 - OnCourse
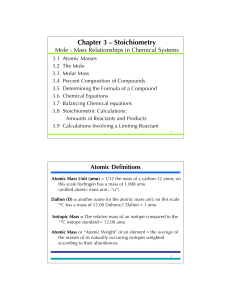
... In the molecule CO2, there is one atom of carbon. The subscript on the symbol for oxygen tells us that there are 2 atoms of oxygen in the molecule. There is one mole of carbon in CO2 There are two moles of oxygen in CO2 The molar mass of CO2 is 1 x 12.01 g C / mol CO2 ...
... In the molecule CO2, there is one atom of carbon. The subscript on the symbol for oxygen tells us that there are 2 atoms of oxygen in the molecule. There is one mole of carbon in CO2 There are two moles of oxygen in CO2 The molar mass of CO2 is 1 x 12.01 g C / mol CO2 ...
From (2)
... Factors affected on its increase Both thermodynamics and kinetics are necessary for proper understanding of metallurgical processes ...
... Factors affected on its increase Both thermodynamics and kinetics are necessary for proper understanding of metallurgical processes ...
- Catalyst
... Problem: Sucrose (C12H22O11) is common table sugar. ( a) What is the mass percent of each element in sucrose? ( b) How many grams of carbon are in 24.35 g of sucrose? (a) Determining the mass percent of each element: mass of C per mole sucrose = 12 x 12.01 g C/mol = 144.12 g C/mol mass of H / mol = ...
... Problem: Sucrose (C12H22O11) is common table sugar. ( a) What is the mass percent of each element in sucrose? ( b) How many grams of carbon are in 24.35 g of sucrose? (a) Determining the mass percent of each element: mass of C per mole sucrose = 12 x 12.01 g C/mol = 144.12 g C/mol mass of H / mol = ...
CH_3 IN TOTO - WordPress.com
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
... Atomic mass is the • weighted average of all naturally occurring isotopes of that element • number on the periodic table below the chemical symbol with two decimal places ...
Chapter 09 An Overview of Chemical Reactions Notes
... Balancing Chemical Equation: - the process by which we place coefficient (numbers in front of reactants and products) in an attempt to equate the number of atoms or polyatomic ions for the elements or compounds in a chemical equation. Steps involve to Balance Chemical Equation 1. Write the chemical ...
... Balancing Chemical Equation: - the process by which we place coefficient (numbers in front of reactants and products) in an attempt to equate the number of atoms or polyatomic ions for the elements or compounds in a chemical equation. Steps involve to Balance Chemical Equation 1. Write the chemical ...
Chem12 SM Unit 5 Review final ok
... 42. (a) In P2O5, the oxidation number of O is –2 and the oxidation number of P is +5. (b) In NO2, the oxidation number of O is –2 and the oxidation number of N is +4. (c) In Na2SO4, the oxidation number of Na is +1, the oxidation number of O is –2, and the oxidation number of S is +6. (d) In Cu(NO3) ...
... 42. (a) In P2O5, the oxidation number of O is –2 and the oxidation number of P is +5. (b) In NO2, the oxidation number of O is –2 and the oxidation number of N is +4. (c) In Na2SO4, the oxidation number of Na is +1, the oxidation number of O is –2, and the oxidation number of S is +6. (d) In Cu(NO3) ...
8.3 Bonding Theories - Chemistry with Mr. Saval
... • sigma bond ( bond): a bond formed when two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting the two atomic nuclei • pi bond ( bond): a covalent bond in which the bonding electrons are most likely to be found in sausage-shaped regions above and be ...
... • sigma bond ( bond): a bond formed when two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting the two atomic nuclei • pi bond ( bond): a covalent bond in which the bonding electrons are most likely to be found in sausage-shaped regions above and be ...
Answer
... • In the spaces provided, explain the meanings of the following terms. You may use an equation or diagram where appropriate. (a) hydrogen bonding An unusually strong dipole-dipole interaction that forms when a hydrogen atom is bonded to one of the very electronegative atoms F, O or N. ...
... • In the spaces provided, explain the meanings of the following terms. You may use an equation or diagram where appropriate. (a) hydrogen bonding An unusually strong dipole-dipole interaction that forms when a hydrogen atom is bonded to one of the very electronegative atoms F, O or N. ...
c00kieee - Ritter Illustration
... initial treatment is performed on bores with a slightly acidic and high oxygenated aqueous solution that is raised to the surface and allowed to go through an extraction process to remove the uranium. No matter which mining process is used, the final U3 O8 is converted to UF6 through a multistep pro ...
... initial treatment is performed on bores with a slightly acidic and high oxygenated aqueous solution that is raised to the surface and allowed to go through an extraction process to remove the uranium. No matter which mining process is used, the final U3 O8 is converted to UF6 through a multistep pro ...
2 H 2
... of product is the limiting reactant. 3. The number of moles of product produced by the limiting reactant is ALL the product possible. There is no more limiting reactant left. ...
... of product is the limiting reactant. 3. The number of moles of product produced by the limiting reactant is ALL the product possible. There is no more limiting reactant left. ...
enthalpy change
... • First it is important to be familiar with some common definitions • The standard molar enthalpy change of formation of a compound, is the heat energy absorbed or released when 1 mol of compound is formed from its elements in their standard states. • Values of can be found on the table of standard ...
... • First it is important to be familiar with some common definitions • The standard molar enthalpy change of formation of a compound, is the heat energy absorbed or released when 1 mol of compound is formed from its elements in their standard states. • Values of can be found on the table of standard ...
Solution - gearju.com
... percentage can be converted directly to grams. In this sample, there will be 40.92 g of C, 4.58 g of H, and 54.50 g of O. Because the subscripts in the formula represent a mole ratio, we need to convert the grams of each element to moles. The conversion factor needed is the molar mass of each elemen ...
... percentage can be converted directly to grams. In this sample, there will be 40.92 g of C, 4.58 g of H, and 54.50 g of O. Because the subscripts in the formula represent a mole ratio, we need to convert the grams of each element to moles. The conversion factor needed is the molar mass of each elemen ...
Harvard University General Chemistry Practice Problems “The
... 2. Add 50. mL of 0.100 M AgNO3 ; a precipitate of AgCl is formed. 3. Add 50. mL of 0.100 M H2 SO4 ; a precipitate of BaSO4 is formed. 4. Finally, add 250. mL of 0.100 M NH3 to neutralize the acid. Determine the concentrations of each of the following species in the resulting mixture: Ba2+ , Cl– , NO ...
... 2. Add 50. mL of 0.100 M AgNO3 ; a precipitate of AgCl is formed. 3. Add 50. mL of 0.100 M H2 SO4 ; a precipitate of BaSO4 is formed. 4. Finally, add 250. mL of 0.100 M NH3 to neutralize the acid. Determine the concentrations of each of the following species in the resulting mixture: Ba2+ , Cl– , NO ...
College Grossmont 115
... or numbers obtained by definition. For example, we can count the fingers on our hand and get an exact number (most people have 5). There is no uncertainty in this result, but we cannot count large groups of objects without some degree of uncertainty. For example, the number of stars in our galaxy is ...
... or numbers obtained by definition. For example, we can count the fingers on our hand and get an exact number (most people have 5). There is no uncertainty in this result, but we cannot count large groups of objects without some degree of uncertainty. For example, the number of stars in our galaxy is ...
Chapter 2 Atoms, molecules and ions
... nucleus to be chemical processes. The postulate is still useful. A slightly more restrictive wording is "Atoms cannot be created, destroyed, or transformed into other atoms in a chemical change". ...
... nucleus to be chemical processes. The postulate is still useful. A slightly more restrictive wording is "Atoms cannot be created, destroyed, or transformed into other atoms in a chemical change". ...
Analytical Chemistry - University of Delhi
... Analytical Chemistry is an applied, experimental field of science and is based not only on chemistry, but also on physics, biology, information theory and many fields of technology. It is of fundamental importance not only to all branches of chemistry but also to all biological sciences, engineering ...
... Analytical Chemistry is an applied, experimental field of science and is based not only on chemistry, but also on physics, biology, information theory and many fields of technology. It is of fundamental importance not only to all branches of chemistry but also to all biological sciences, engineering ...
Periodic table, elements and physical chemistry
... identify and contact all copyright holders whose work is used in this paper. To avoid the issue of disclosure of answer-related information to candidates, all copyright acknowledgements are reproduced in the OCR Copyright Acknowledgements booklet. This is produced for each series of examinations and ...
... identify and contact all copyright holders whose work is used in this paper. To avoid the issue of disclosure of answer-related information to candidates, all copyright acknowledgements are reproduced in the OCR Copyright Acknowledgements booklet. This is produced for each series of examinations and ...
Theories of the constitution of gases in the early nineteenth century
... to consolidate the error of both. The difference between the theories always lay in simple numerical ratios. The correct theory, which acknowledged the equal apparent size of all gas particles, was thought incapable of giving atomic weight values and was ignored until it was found useful. This thesi ...
... to consolidate the error of both. The difference between the theories always lay in simple numerical ratios. The correct theory, which acknowledged the equal apparent size of all gas particles, was thought incapable of giving atomic weight values and was ignored until it was found useful. This thesi ...