* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemical Reactions
Isotopic labeling wikipedia , lookup
Organic chemistry wikipedia , lookup
Chemical element wikipedia , lookup
History of chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Stoichiometry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Atomic theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
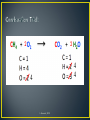
Chapter 11 L. Bernard, 2015 L. Bernard, 2015 Do Now! L. Bernard, 2015 • The process by which atoms of the same or different element rearrange themselves to form a new substance • Bonds are broken between atoms and re-formed to form new molecules reactants products L. Bernard, 2015 • • • • • • • Production of heat Absorption of heat (gets cold) Production of gas (bubbles) Production of a precipitate (solid) Change in color Change in odor Change in state of matter L. Bernard, 2015 1. 2. 3. 4. 5. Synthesis Decomposition Single-Replacement Double-Replacement Combustion L. Bernard, 2015 • Also known as combination • When two or more substances react to form a SINGLE substance L. Bernard, 2015 L. Bernard, 2015 • When a single substance breaks down into two or more products • Opposite of synthesis L. Bernard, 2015 L. Bernard, 2015 • Where one element replaces another element in a compound • Essentially one element switches compounds L. Bernard, 2015 L. Bernard, 2015 • When there is an exchange of POSITIVE IONS between two compounds • The cations exchange places L. Bernard, 2015 L. Bernard, 2015 • Where an element or compound reacts with oxygen to produce carbon dioxide and water • Produces heat and light L. Bernard, 2015 L. Bernard, 2015 • P4 + 3O2 2P2O3 • Synthesis! • 2MgI2 + Mn(SO3)2 2MgSO3 + MnI4 • Double Displacement! • C6H12 + 9O2 6CO2 + 6H2O • Combustion! • 2AgNO3 + Cu Cu(NO3)2 + 2Ag • Single Displacement! L. Bernard, 2015 • • • • • • • • Pb + FeSO4 PbSO4 + Fe P4 + 3 O2 2 P2O3 C6H12 + 9 O2 6 CO2 + 6 H2O O3 O. + O2 2 AgNO3 + Cu Cu(NO3)2 + 2 Ag 2 MgI2 + Mn(SO3)2 2 MgSO3 + MnI4 2 NO2 2 O2 + N2 SeCl6 + O2 SeO2 + 3Cl2 L. Bernard, 2015 L. Bernard, 2015 • Law of Conservation of Mass • Matter cannot be created nor destroyed • Just like with math, both sides of the YIELDS sign must be equal! • So the same number of atoms must be on both sides of the equation L. Bernard, 2015 L. Bernard, 2015 1. Inspection 2. “ABC” Method 3. Combustion Trick L. Bernard, 2015 • Use when it is easy to see the solution 1. List all the elements within the chemical equation 2. Count the number of elements on each side 3. Balance the coefficients and charges L. Bernard, 2015 L. Bernard, 2015 • Algebraically solve for the coefficients 1. Assign a letter for each coefficient in the reaction 2. Determine an algebraic equation to solve for the value of each coefficient L. Bernard, 2015 L. Bernard, 2015 L. Bernard, 2015 L. Bernard, 2015 • Use only with combustion reactions! 1. Balance the carbon and hydrogen first using the inspection method 2. Balance the number of oxygens last • In the reactants, oxygen will always be alone as O2 L. Bernard, 2015 2 2 4 4 4 L. Bernard, 2015 •Li + H2O → LiOH + H2 •K + B2O3 → K2O + B •C6H6 + O2 → CO2 + H2O L. Bernard, 2015 • Matter can be changed from one form to another • Cannot be created or destroyed! • Why must we balance equations? L. Bernard, 2015