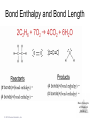* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 8 Concepts of Chemical Bonding
Chemical industry wikipedia , lookup
Atomic orbital wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Chemical potential wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
History of chemistry wikipedia , lookup
Hydrogen bond wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Bent's rule wikipedia , lookup
Metalloprotein wikipedia , lookup
Halogen bond wikipedia , lookup
Electron configuration wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Electronegativity wikipedia , lookup
Atomic theory wikipedia , lookup
Bond valence method wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
History of molecular theory wikipedia , lookup
Metallic bonding wikipedia , lookup
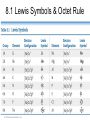
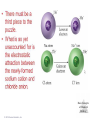
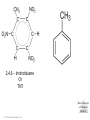
Lecture Presentation Chapter 8 Basic Concepts of Chemical Bonding (With Chapter 9) MC 7 out of 60 FRQ every year James F. Kirby Quinnipiac University Hamden, CT © 2015 Pearson Education, Inc. If only noble gases are stable as atoms, how do other atoms achieve stability? Chemical Bonds © 2015 Pearson Education, Inc. 8.1 Chemical Bonds, Lewis Symbols and the Octet Rule © 2015 Pearson Education, Inc. • Three basic types of bonds – Ionic • Electrostatic attraction between ions. • Metal-nonmetal – Covalent • Sharing of electrons. • Nonmetal-nonmetal – Metallic • Metal atoms bonded Basic to Concepts Chemical several other atoms. ofBonding • Metal-metal All chemical bonds form because the electron-proton attraction lowers the potential energy of the system as the bond forms. The distance between the bonded particles is a balance between the attractions of the oppositely charged particles and repulsions of the like charged particles. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. 8.1 Lewis Symbols & Octet Rule Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. • G. N. Lewis developed a method to denote potential bonding electrons by using one dot for every valence electron around the element symbol. • When forming compounds, atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons - electrons involved in bonding (the octet rule). - An octet consists of full s and p subshells. - We know that s2p6 is a noble gas configuration. - We assume that an atom is stable when surrounded by 8 e-’s ( four electron pairs) * Elements 1-5 are too small to form a full octet. Basic Concepts * Some larger non-metallic (Period 3+) atoms can of Chemical Bonding expand their valence shell to 10 to 12 electrons. © 2015 Pearson Education, Inc. Are all these Lewis symbols for Cl correct? a. Yes, all three are correct. b. No, the first two are different Lewis structures because of the differing placement of unpaired electrons. c. No, because the Lewis structure on the right has only six valence electrons and elemental Cl has seven valence electrons. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. 8.2 Ionic Formation • Atoms tend to lose (metals) or gain (nonmetals) electrons to have a noble gas electron configuration. • When forming ionic bonds, like when NaCl is formed from Na and Cl, Cl removes an e- from the Na and ionic bond forms. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Describe the electron transfers that occur in the formation of calcium fluoride from elemental calcium and elemental fluorine. a. Each calcium atom loses one electron and each fluorine atom gains two electrons. b. Each calcium atom loses two electrons and each fluorine atom gains one electron. c. Each calcium atom gains one electron and each fluorine atom loses two electrons. d. Each calcium atom gains two electrons and each fluorine atom loses one electron. © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Energetics of Ionic Bonding Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Lattice Energy • This third piece of the puzzle is the lattice energy, – the energy required to completely separate a mole of a solid ionic compound into its gaseous ions. • It is a measure of the strength of the electrostatic attraction that is the principle cause of ionic compound stability. • The energy associated with electrostatic interactions is governed by Coulomb’s law: Q 1Q 2 Eel = d2 © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Lattice Energy • Lattice energy increases with: – increasing charge on the ions – decreasing size of ions Q 1Q 2 Eel = d2 © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Energetics of Ionic Bonding— Born-Haber Cycle • By accounting for all three energies (ionization energy, electron affinity and lattice energy), we can get a good idea of the energetics involved in such a process. • Start with the metal and nonmetal elements: Na(s) and Cl2(g). • Make gaseous atoms: Na(g) and Cl(g). • Make ions: Na+(g) and Cl–(g). • Combine the ions: NaCl(s). © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. If you were to perform the reaction KCl(s) ⟶ K+ (g) + Cl–(g), would energy be released? a. Yes, the separation of the ions causes the release of energy. b. No, the separation of the ions requires energy. c. No, the separation of ions does not cause any change in energy. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.1 Magnitude of Lattice Energies Without consulting Table 8.2, arrange the ionic compounds NaF, CsI, and CaO in order of Increasing lattice energy. Solution Analyze From the formulas for three ionic compounds, we must determine their relative lattice energies. Plan We need to determine the charges and relative sizes of the ions in the compounds. We then use Equation 8.4 qualitatively to determine the relative energies, knowing that (a) the larger the ionic charges, the greater the energy and (b) the farther apart the ions are, the lower the energy. Solve NaF consists of Na+ and F− ions, CsI of Cs+ and I− ions, and CaO of Ca2+ and O2− ions. Because the product Q1Q2 appears in the numerator of Equation 8.4, the lattice energy increases dramatically when the charges increase. Thus, we expect the lattice energy of CaO, which has 2+ and 2− ions, to be the greatest of the three. The ionic charges are the same in NaF and CsI. The difference in their lattice energies thus depends on the difference in the distance between ions in the lattice. Because ionic size increases as we go down a group in the periodic table (Section 7.3), we know that Cs+ is larger than Na+ and I− is larger than F−. Therefore, the distance between Na+ and F− ions in NaF is less than the distance between the Cs+ and I− ions in CsI. As a result, the lattice energy of NaF should be greater than that of CsI. In order of increasing energy, therefore, we have CsI < NaF < CaO. Check Table 8.2 confirms our predicted order is correct. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.1 Magnitude of Lattice Energies Practice Exercise 1 Without looking at Table 8.2, predict which one of the following orderings of lattice energy is correct for these ionic compounds. (a) NaCl > MgO > CsI > ScN, (b) ScN > MgO > NaCl > CsI, (c) NaCl > CsI > ScN > CaO, (d) MgO > NaCl > ScN > CsI, (e) ScN > CsI > NaCl > MgO. Practice Exercise 2 Which substance do you expect to have the greatest lattice energy: MgF2, CaF2, or ZrO2? Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.2 Charges on Ions Predict the ion generally formed by (a) Sr, (b) S, (c) Al. Solution Analyze We must decide how many electrons are most likely to be gained or lost by atoms of Sr, S, and Al. Plan In each case we can use the element’s position in the periodic table to predict whether the element forms a cation or an anion. We can then use its electron configuration to determine the most likely ion formed. Solve (a) Strontium is a metal in group 2A and therefore forms a cation. Its electron configuration is [Kr]5s2, and so we expect that the two valence electrons will be lost to give an Sr2+ ion. (b) Sulfur is a nonmetal in group 6A and will thus tend to be found as an anion. Its electron configuration ([Ne]3s23p4) is two electrons short of a noble-gas configuration. Thus, we expect that sulfur will form S2– ions. (c) Aluminum is a metal in group 3A. We therefore expect it to form Al3+ ions. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.2 Charges on Ions Continued Check The ionic charges we predict here are confirmed in Tables 2.4 and 2.5. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.2 Charges on Ions Continued Practice Exercise 1 Which of these elements is most likely to form ions with a 2+ charge? (a) Li, (b) Ca, (c) O, (d) P, (e) Cl. Practice Exercise 2 Predict the charges on the ions formed when magnesium reacts with nitrogen. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. 8.3 Covalent Bonding • In covalent bonds, atoms share electrons. • There are several electrostatic interactions in these bonds: – attractions between electrons and nuclei, – repulsions between electrons, and – repulsions between nuclei. • For a bond to form, the attractions must be greater than the repulsions. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Q: What would happen to the magnitudes of the attractions and repulsions represented in (a) if the nuclei were farter apart? Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Ionizing an H2 molecule to H2+ changes the strength of the bond. Based on the description of covalent bonding given previously, do you expect the H—H bond in H2+ to be weaker or stronger than the H—H bond in H2? a. Stronger, because a H—H covalent bond in H2+ has one less electron than in H2. b. Stronger, because a H—H covalent bond in H2+ has one more electron than in H2. c. Weaker, because a H—H covalent bond in H2+ has one less electron than in H2. d. Weaker, because a H—H covalent bond in H2+ has one Basic Concepts more electron than in H2. of Chemical Bonding © 2015 Pearson Education, Inc. Lewis Structures • By sharing valence electrons, atoms in a molecular compound can achieve a noble-gas electron configuration. • The simplest examples are for hydrogen, H2, and chlorine, Cl2, shown below. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Electrons on Lewis Structures • Lone pairs(unshared pair): pairs of electrons that is not being shared or located on only one atom in a Lewis structure • Bonding pairs(shared pairs): shared electrons in a Lewis structure; they can be represented by a line Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Multiple Bonds • Single covalent bond – sharing of 1 pair of electron between atoms. H-Cl • NOTE: Atoms of C, N, O and sometimes S can form multiple bonds. • Double covalent bonds – sharing of 2 pairs of electrons between atoms. • Triple covalent bonds - sharing of 3 pairs of electrons between atoms. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.3 Lewis Structure of a Compound Given the Lewis symbols for nitrogen and fluorine in Table 8.1, predict the formula of the stable binary compound (a compound composed of two elements) formed when nitrogen reacts with fluorine and draw its Lewis structure. Solution Analyze The Lewis symbols for nitrogen and fluorine reveal that nitrogen has five valence electrons and fluorine has seven. Plan We need to find a combination of the two elements that results in an octet of electrons around each atom. Nitrogen requires three additional electrons to complete its octet, and fluorine requires one. Sharing a pair of electrons between one N atom and one F atom will result in an octet of electrons for fluorine but not for nitrogen. We therefore need to figure out a way to get two more electrons for the N atom. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.3 Lewis Structure of a Compound Continued Solve Nitrogen must share a pair of electrons with three fluorine atoms to complete its octet. Thus, the binary compound these two elements form must be NF3: Check The Lewis structure in the center shows that each atom is surrounded by an octet of electrons. Once you are accustomed to thinking of each line in a Lewis structure as representing two electrons, you can just as easily use the structure on the right to check for octets. Practice Exercise 1 Which of these molecules has the same number of shared electron pairs as unshared electron pairs? (a) HCl, (b) H2S, (c) PF3, (d) CCl2F2 (e) Br2. Practice Exercise 2 Compare the Lewis symbol for neon with the Lewis structure for methane, CH4. How many valence electrons are in each structure? How many bonding pairs and how many nonbonding pairs does each Basic Concepts of Chemical structure have? Bonding © 2015 Pearson Education, Inc. The C—O bond length in carbon monoxide, CO, is 1.13 Å, whereas the C—O bond length in CO2 is 1.24 Å. Without drawing a Lewis structure, do you think that CO contains a single, double, or triple bond? a. Single covalent bond b. Double covalent bond c. Triple covalent bond Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. 8.4 Polar Covalent Bonds • The electrons in a covalent bond are not always shared equally. • Fluorine pulls harder on the electrons it shares with hydrogen than hydrogen does. • Therefore, the fluorine end of the molecule has more electron density than the hydrogen end. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Bond Polarity Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Electronegativity • Electronegativity is the ability of an atom in a molecule to attract electrons to itself. • On the periodic table, electronegativity generally increases as you go – from left to right across a period (>Zeff). – from the bottom to the top of a group (<n). Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Electronegativity and Bond Polarity The difference in electronegativity between two atoms in a bond determine the bond polarity. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Q: For the group 6A elements, what is the trend in electronegativity with increasing atomic number? Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Electronegativity and Polar Covalent Bonds • When two atoms share electrons unequally, a polar covalent bond results. • Electrons tend to spend more time around the more electronegative atom. The result is a partial negative charge (not a complete transfer of charge). It is represented by δ–. • The other atom is “more positive,” or δ+. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Polar Covalent Bonds The greater the difference in electronegativity, the more polar is the bond. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Based on differences in electronegativity, how would you characterize the bonding in sulfur dioxide, SO2? Do you expect the bonds between S and O to be nonpolar, polar covalent, or ionic? a. Nonpolar, because both S and O are in the same family. b. Polar covalent, because the difference in electronegativity values is 1.0. c. Ionic, because the difference in electronegativity values is –1.0. d. Ionic, because the difference in electronegativity values is >0.9. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.4 Bond Polarity In each case, which bond is more polar? (a) B—Cl or C—Cl, (b) P—F or P—Cl. Indicate in each case which atom has the partial negative charge. Solution Analyze We are asked to determine relative bond polarities, given nothing but the atoms involved in the bonds. Plan Because we are not asked for quantitative answers, we can use the periodic table and our knowledge of electronegativity trends to answer the question. Solve (a) The chlorine atom is common to both bonds. Therefore, we just need to compare the electronegativities of B and C. Because boron is to the left of carbon in the periodic table, we predict that boron has the lower electronegativity. Chlorine, being on the right side of the table, has high electronegativity. The more polar bond will be the one between the atoms with the biggest differences in electronegativity. Consequently, the B—Cl bond is more polar; the chlorine atom carries the partial negative charge because it has a higher electronegativity. (b) In this example phosphorus is common to both bonds, and so we just need to compare the electronegativities of F and Cl. Because fluorine is above chlorine in the periodic table, it should be more electronegative and will form the more polar bond with P. The higher electronegativity of fluorine means that it will carry the partial negative charge. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.4 Bond Polarity Continued Check (a) Using Figure 8.7: The difference in the electronegativities of chlorine and boron is 3.0 – 2.0 = 1.0; the difference between the electronegativities of chlorine and carbon is 3.0 – 2.5 = 0.5.Hence, the B—Cl bond is more polar, as we had predicted. (b) Using Figure 8.7: The difference in the electronegativities of chlorine and phosphorus is 3.0 – 2.1 = 0.9; the difference between the electronegativities of fluorine and phosphorus is 4.0 – 2.1 = 1.9. Hence, the P—F bond is more polar, as we had predicted. Practice Exercise 1 Which of the following bonds is the most polar? (a) H—F, (b) H—I, (c) Se—F, (d) N—P, (e) Ga—Cl. Practice Exercise 2 Which of the following bonds is most polar: S—Cl, S—Br, Se—Cl, or Se—Br? Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Is a Compound Ionic or Covalent? • Simplest approach: Metal + nonmetal is ionic; nonmetal + nonmetal is covalent. • There are many exceptions: It doesn’t take into account oxidation number of a metal (higher oxidation numbers can give covalent bonding). • Electronegativity difference can be used; the table still doesn’t take into account oxidation number. • Properties of compounds are often best: Lower melting points mean covalent bonding, for example. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. 8.5 Drawing Lewis Structures (Covalent Molecules) Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. 8.5 Drawing Lewis Structures (Covalent Molecules) PCl3 Always keep track of the electrons: 5 + 3(7) = 26 1. Find the sum of the valence electrons of all atoms in the polyatomic ion or molecule, – If it is an anion, add one electron for each negative charge. – If it is a cation, subtract one electron for each positive charge. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Drawing Lewis Structures 2. The central atom is the least electronegative element that isn’t H. Connect the outer atoms to it by single bonds (a line representing two electrons). Keep track of the electrons: 26 − 6 = 20 © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Drawing Lewis Structures 3. Fill the octets of the outer atoms. Keep track of the electrons: 26 − 6 = 20; 20 − 18 = 2 © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Drawing Lewis Structures 4. Fill the octet of the central atom. Keep track of the electrons: 26 − 6 = 20; 20 − 18 = 2; 2 − 2 = 0 © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Drawing Lewis Structures 5. If you run out of electrons before the central atom has an octet, form multiple bonds until it does. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Drawing Lewis Structures 6. If you have extra electrons, place them as pairs on the central atom. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.8 Lewis Structure for a Polyatomic Ion Draw the Lewis structure for the BrO ion. – 3 Solution Bromine (group 7A) has seven valence electrons, and oxygen (group 6A) has six. We must add one more electron to our sum to account for the 1– charge of the ion. The total number of valence electrons is, therefore, 7 + (3 ✕ 6) + 1 = 26. For oxyanions — SO42–, NO3–, CO32–, and so forth — the oxygen atoms surround the central nonmetal atom. After arranging the O atoms around the Br atom, drawing single bonds, and distributing the unshared electron pairs, we have Notice that the Lewis structure for an ion is written in brackets and the charge is shown outside the brackets at the upper right. Practice Exercise 1 How many nonbonding electron pairs are there in the Lewis structure of the peroxide ion, O22–? (a) 7, (b) 6, (c) 5, (d) 4, (e) 3. Practice Exercise 2 Draw the Lewis structure for (a) ClO2–, (b) PO43–. © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Formal Charge & Alternative Lewis Structure • Then assign formal charges. • Formal charge is the charge an atom would have if all of the electrons in a covalent bond were shared equally. • Formal charge = valence electrons – ½ (bonding electrons) – all nonbonding electrons • The best Lewis structure is the one with the fewest charges. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Drawing Lewis Structures • The dominant Lewis structure – is the one in which atoms have formal charges closest to zero. – puts a negative formal charge on the most electronegative atom. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Suppose a Lewis structure for a neutral fluorinecontaining molecule results in a formal charge of +1 on the fluorine atom. What conclusion would you draw? a. The structure actually represents an ion. b. The F atom in the structure must have four covalent bonds attached to it. c. There must be another F atom in the structure carrying a –1 formal charge, since F is the most electronegative element and it should carry a negative formal charge. d. There must be a better Lewis structure, since F is the most electronegative element and it should carry a negative formal charge. © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Sample Exercise 8.9 Lewis Structures and Formal Charges Three possible Lewis structures for the thiocyanate ion, NCS–, are (a) Determine the formal charges in each structure. (b) Based on the formal charges, which Lewis structure is the dominant one? Solution (a) Neutral N, C, and S atoms have five, four, and six valence electrons, respectively. We can determine the formal charges in the three structures by using the rules we just discussed: As they must, the formal charges in all three structures sum to 1–, the overall charge of the ion. (b) The dominant Lewis structure generally produces formal charges of the smallest magnitude (guideline 1). That eliminates the left structure as the dominant one. Further, as discussed in Section 8.4, N is more electronegative than C or S. Therefore, we expect any negative formal charge to reside on the N atom (guideline 2). For these two reasons, the middle Lewis structure is the dominant one for NCS–. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.9 Lewis Structures and Formal Charges Continued Practice Exercise 1 Phosphorus oxychloride has the chemical formula POCl3, with P as the central atom. To minimize formal charge, how many bonds does phosphorus make to the other atoms in the molecule? (Count each single bond as one, each double bond as two, and each triple bond as three.) (a) 3, (b) 4, (c) 5, (d) 6, (e) 7 Practice Exercise 2 The cyanate ion, NCO–, has three possible Lewis structures. (a) Draw these three structures and assign formal charges in Basic Concepts each. (b) Which Lewis structure is dominant? of Chemical Bonding © 2015 Pearson Education, Inc. 8.6 Resonance Structures The Best Lewis Structure? • Following our rules, this is the Lewis structure we would draw for ozone, O3. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. • One Lewis structure cannot accurately depict a molecule like ozone. • We use multiple structures, resonance structures, to describe the molecule. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. The O—O bonds in ozone are often described as “one-and-a-half” bonds. Is this description consistent with the idea of resonance? a. Yes, because in each of the two resonance forms there is one O=O bond and one O—O bond, giving an overall average of 1.5 bonds per O—O bond. b. No, because a half bond cannot exist in a bonding situation. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. In the same sense that we describe the O—O bonds in O3 as “one-and-a-half” bonds, how would you describe the N—O bonds in NO3–? a. One-and-a-fifth bonds b. One-and-a-fourth bonds c. One-and-a-third bonds d. One-and-a-half bonds Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.10 Resonance Structures Which is predicted to have the shorter sulfur–oxygen bonds, SO3 or SO32–? Solution The sulfur atom has six valence electrons, as does oxygen. Thus, SO 3 contains 24 valence electrons. In writing the Lewis structure, we see that three equivalent resonance structures can be drawn: As with NO3– the actual structure of SO3 is an equal blend of all three. Thus, each S—O bond length should be about one-third of the way between the length of a single bond and the length of a double bond. That is, S—O should be shorter than single bonds but not as short as double bonds. The SO32 ion has 26 electrons, which leads to a dominant Lewis structure in which all the S—O bonds are single: Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.10 Resonance Structures Continued Our analysis of the Lewis structures thus far leads us to conclude that SO 3 should have the shorter S—O bonds and SO32– the longer ones. This conclusion is correct: The experimentally measured S—O bond lengths are 1.42 Å in SO3 and 1.51 Å in SO32–. Practice Exercise 1 Which of these statements about resonance is true? (a) When you draw resonance structures, it is permissible to alter the way atoms are connected. (b) The nitrate ion has one long N—O bond and two short N—O bonds. (c) “Resonance” refers to the idea that molecules are resonating rapidly between different bonding patterns. (d) The cyanide ion has only one dominant resonance structure. (e) All of the above are true. Practice Exercise 2 Draw two equivalent resonance structures for the formate ion, HCO2–. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Resonance in Benzene • The organic compound benzene, C6H6, has two resonance structures. • It is commonly depicted as a hexagon with a circle inside to signify the delocalized electrons in the ring. Localized electrons are specifically on one atom or shared between two atoms; Delocalized electrons are shared by multiple atoms. © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding What do aspirin, Tylenol, ibuprofen, almonds, vanilla and mothballs have in common? Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. 2,4,6 – trinitrotoluene Or TNT Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Each Lewis structure of benzene has three C=C double bonds. Another hydrocarbon containing three C=C double bonds is hexatriene, C6H8. A Lewis structure of hexatriene is Do you expect hexatriene to have multiple resonance structures? If not, why is this molecule different from benzene with respect to resonance? a. Yes, because it has multiple Lewis resonance structures. b. Yes, because it has three C=C bonds that can be moved throughout the structure. c. No, because the carbon chain is linear. d. Basic Concepts No, because we cannot write other reasonable Lewis structures. of Chemical Bonding © 2015 Pearson Education, Inc. 8.7 Exceptions to the Octet Rule • There are three types of ions or molecules that do not follow the octet rule: – ions or molecules with an odd number of electrons, – ions or molecules with less than an octet, – ions or molecules with more than eight valence electrons (an expanded octet). Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Odd Number of Electrons Though relatively rare and usually quite unstable and reactive, there are ions and molecules with an odd number of electrons. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Which of the Lewis structures for NO is dominant based on analysis of the formal charges? a. The first NO structure, because all atoms have zero formal charge. b. The first NO structure, because N and O have equal but opposite formal charges. c. The second NO structure, because all atoms have zero formal charge. d. The second NO structure, because N should not have a Basic Concepts positive formal charge. of Chemical Bonding © 2015 Pearson Education, Inc. Fewer Than Eight Electrons • Elements in the second period before carbon can make stable compounds with fewer than eight electrons. • Consider BF3: – Giving boron a filled octet places a negative charge on the boron and a positive charge on fluorine. – This would not be an accurate picture of the distribution of electrons in BF3. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Fewer Than Eight Electrons The lesson is: If filling the octet of the central atom results in a negative charge on the central atom and a positive charge on the more electronegative outer atom, don’t fill the octet of the central atom. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. • Q: Draw the lewis structure for BeF2 Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. More Than Eight Electrons • When an element is in period 3 or below in the periodic table (e.g., periods 3, 4, 5, etc.), it can use d-orbitals to make more than four bonds. • Examples: PF5 and phosphate below (Note: Phosphate will actually have four resonance structures with five bonds on the P atom!) Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. • This eliminates the charges on the phosphorus and the charge on one of the oxygens. • The lesson is: when the central atom is on the 3rd row or below and expanding its octet eliminates some formal charges, do so. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.11 Lewis Structure for an Ion with More Than an Octet of Electrons Draw the Lewis structure for ICl4–. Solution Iodine (group 7A) has seven valence electrons. Each chlorine atom (group 7A) also has seven. An extra electron is added to account for the 1– charge of the ion. Therefore, the total number of valence electrons is 7 + (4 × 7) + 1 = 36. The I atom is the central atom in the ion. Putting eight electrons around each Cl atom (including a pair of electrons between I and each Cl to represent the single bond between these atoms) requires 8 × 4 = 32 electrons. We are thus left with 36 – 32 = 4 electrons to be placed on the larger iodine: Iodine has 12 valence electrons around it, four more than needed for an octet. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.11 Lewis Structure for an Ion with More Than an Octet of Electrons Continued Practice Exercise 1 In which of these molecules or ions is there only one lone pair of electrons on the central sulfur atom? (a) SF4, (b) SF6, (c) SOF4, (d) SF2, (e) SO42–. Practice Exercise 2 (a) Which of the following atoms is never found with more than an octet of valence electrons around it? S, C, P, Br, I. (b) Draw the Lewis structure for XeF2. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. 8.8 Covalent Bond Strength • Most simply, the strength of a bond is measured by determining how much energy is required to break the bond. • This is called the bond enthalpy. • The bond enthalpy for a Cl—Cl bond, D(Cl— Cl), is measured to be 242 kJ/mol. • We write out reactions for breaking one mole of those bonds: Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. How can you use the enthalpy of atomization of the hydrocarbon ethane, C2H6(g), along with the value D(C—H) = 413 kJ/mol to estimate the value for D(C—C)? a. The enthalpy of atomization l 7 bonds broken = a good estimate of D(C—C). b. The enthalpy of atomization – 6 [D(C—H)] = a good estimate of D(C—C). c. The enthalpy of atomization + 6 [D(C—H)] = a good estimate of D(C—C). d. The enthalpy of atomization l 7 bonds broken – 6[D(C—H)] = a good estimate of D(C—C). © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Average Bond Enthalpies • Average bond enthalpies are positive, because bond breaking is an endothermic process. • Note that these are averages over many different compounds; not every bond in nature for a pair of atoms has exactly the same bond energy. © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Average Bond Enthalpies • NOTE: These are average bond enthalpies, not absolute bond enthalpies; the C-H bonds in CH4, will be a bit different than the C-H bond in CHCl3.(Chloroform) Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Based on bond enthalpies, which do you expect to be more reactive, oxygen, O2, or hydrogen peroxide, H2O2? a. O2 is more reactive, because the O=O bond enthalpy is less than that of the O—O bond enthalpy in hydrogen peroxide. b. O2 is more reactive, because the O=O bond enthalpy is greater than that of the O—O bond enthalpy in hydrogen peroxide. c. H2O2 is more reactive, because the O—O bond enthalpy is less than that of the O=O bond enthalpy in O2. d. H2O2 is more reactive, because the O—O bond enthalpy is greater than that of the O=O bond enthalpy in O2. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Using Bond Enthalpies to Estimate Enthalpy of Reaction • One way to estimate H for a reaction is to use the bond enthalpies of bonds broken and the new bonds formed. • Energy is added to break bonds and released when making bonds. • In other words, Hrxn = (bond enthalpies of all bonds broken) − (bond enthalpies of all bonds formed). © 2015 Pearson Education, Inc. Basic Concepts of Chemical Bonding Example From the figure on the last slide CH4(g) + Cl2(g) CH3Cl(g) + HCl(g) • In this example, one C—H bond and one Cl— Cl bond are broken; one C—Cl and one H—Cl bond are formed. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Answer H = [D(C—H) + D(Cl—Cl)] − [D(C—Cl) + D(H—Cl)] = [(413 kJ) + (242 kJ)] − [(328 kJ) + (431 kJ)] = (655 kJ) − (759 kJ) = −104 kJ Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Bond Enthalpy and Bond Length 1. It often helps to draw the Lewis structure because it is easier to see all of the bonds of each type of that are formed and broken. 2. Multiply the number of each bond times its bond energy. 3. Total the reactants and products 4. Energy = reactants - products Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Bond Enthalpy and Bond Length Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.12 Using Average Bond Enthalpies Using data from Table 8.4, estimate ΔH for the combustion reaction Solution Analyze We are asked to estimate the enthalpy change for a chemical reaction by using average bond enthalpies for the bonds broken and formed. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.12 Using Average Bond Enthalpies Continued Plan In the reactants, we must break twelve C—H bonds and two C—C bonds in the two molecules of C2H6 and seven O O bonds in the seven O2 molecules. In the products, we form eight C O bonds (two in each CO2) and twelve O—H bonds (two in each H2O). Solve Using Equation 8.12 and data from Table 8.4, we have ΔH = [12D(C—H) + 2D(C—C) + 7D(O O)] – [8D(C O) + 12D(O—H)] = [12(413 kJ) + 2(348 kJ) + 7(495 kJ)] – [8(799 kJ) + 12(463 kJ)] = 9117 kJ – 11948 kJ = –2831 kJ Check This estimate can be compared with the value of –2856 kJ calculated from more accurate thermochemical data; the agreement is good. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Exercise 8.12 Using Average Bond Enthalpies Continued Practice Exercise 1 Using Table 8.4, estimate ΔH for the “water splitting reaction”: H2O(g) → H2(g) + (a) 242 kJ, (b) 417 kJ, (c) 5 kJ, (d) –5 kJ, (e) –468 kJ O2(g). Practice Exercise 2 Using Table 8.4, estimate ΔH for the reaction Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Bond Enthalpy and Bond Length • We can also measure an average bond length for different bond types. • As the number of bonds between two atoms increases, the bond length decreases. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. • What is the bond length and bond energy for the H2 molecule? Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. • If this diagram represents formation of C-C bond, draw the C=C bond formation on the same graph. Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Integrative Exercise Putting Concepts Together Phosgene, a substance used in poisonous gas warfare during World War I, is so named because it was first prepared by the action of sunlight on a mixture of carbon monoxide and chlorine gases. Its name comes from the Greek words phos (light) and genes (born of). Phosgene has the following elemental composition: 12.14% C, 16.17% O, and 71.69% Cl by mass. Its molar mass is 98.9 g ⁄ mol. (a) Determine the molecular formula of this compound. (b) Draw three Lewis structures for the molecule that satisfy the octet rule for each atom. (The Cl and O atoms bond to C.) (c) Using formal charges, determine which Lewis structure is the dominant one. (d) Using average bond enthalpies, estimate ΔH for the formation of gaseous phosgene from CO(g) and Cl2(g). Solution (a) The empirical formula of phosgene can be determined from its elemental composition. (Section 3.5) Assuming 100 g of the compound and calculating the number of moles of C, O, and Cl in this sample, we have Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Integrative Exercise Putting Concepts Together Continued The ratio of the number of moles of each element, obtained by dividing each number of moles by the smallest quantity, indicates that there is one C and one O for each two Cl in the empirical formula, COCl2. The molar mass of the empirical formula is 12.01 + 16.00 + 2(35.45) = 98.91 g ⁄ mol, the same as the molar mass of the molecule. Thus, COCl2 is the molecular formula. (b) Carbon has four valence electrons, oxygen has six, and chlorine has seven, giving 4 + 6 + 2(7) = 24 electrons for the Lewis structures. Drawing a Lewis structure with all single bonds does not give the central carbon atom an octet. Using multiple bonds, three structures satisfy the octet rule: (c) Calculating the formal charges on each atom gives Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc. Sample Integrative Exercise Putting Concepts Together Continued (d) Writing the chemical equation in terms of the Lewis structures of the molecules, we have Thus, the reaction involves breaking a C O bond and a Cl—Cl bond and forming a C=O bond and two C—Cl bonds. Using bond enthalpies from Table 8.4, we have ΔH = [D(C O) + D(Cl – Cl)] – [D(C=O) + 2D(C—Cl)] = [1072 kJ + 242 kJ] – [799 kJ + 2(328 kJ)] = –141 kJ Notice that the reaction is exothermic. Nevertheless, energy is needed from sunlight or another source for the reaction to begin, as is the case for the combustion of H2(g) and O2(g) to form H2O(g) (Figure 5.14). Basic Concepts of Chemical Bonding © 2015 Pearson Education, Inc.