Chemistry 101L
... will be making. Remember to include room for multiple trials and average values, if appropriate. If appropriate, have room for classmates’ data. Now organize your list into things that are similar or data that should be compared. Tables columns/rows do not have to be listed in the same order that th ...
... will be making. Remember to include room for multiple trials and average values, if appropriate. If appropriate, have room for classmates’ data. Now organize your list into things that are similar or data that should be compared. Tables columns/rows do not have to be listed in the same order that th ...
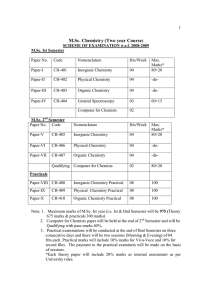
M.Sc. Chemistry (Two year Course)
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
Specification – AS/A Level Chemistry A
... These specifications have been developed for students who wish to continue with a study of chemistry at Level 3 in the National Qualifications Framework (NQF). The AS specification has been written to provide progression from GCSE Science and GCSE Additional Science, or from GCSE Chemistry; achievem ...
... These specifications have been developed for students who wish to continue with a study of chemistry at Level 3 in the National Qualifications Framework (NQF). The AS specification has been written to provide progression from GCSE Science and GCSE Additional Science, or from GCSE Chemistry; achievem ...
Chapter 6 - Suffolk County Community College
... • The rather high specific heat of water allows water to absorb a lot of heat energy without a large increase in its temperature • The large amount of water absorbing heat from the air keeps beaches cool in the summer without water, the Earth’s temperature would be about the same as the moon’s tem ...
... • The rather high specific heat of water allows water to absorb a lot of heat energy without a large increase in its temperature • The large amount of water absorbing heat from the air keeps beaches cool in the summer without water, the Earth’s temperature would be about the same as the moon’s tem ...
Chapter 6 Thermochemistry - Suffolk County Community College
... • The rather high specific heat of water allows water to absorb a lot of heat energy without a large increase in its temperature • The large amount of water absorbing heat from the air keeps beaches cool in the summer without water, the Earth’s temperature would be about the same as the moon’s tem ...
... • The rather high specific heat of water allows water to absorb a lot of heat energy without a large increase in its temperature • The large amount of water absorbing heat from the air keeps beaches cool in the summer without water, the Earth’s temperature would be about the same as the moon’s tem ...
Packet 1 - Kentucky Community and Technical College System
... precipitate forms a , that then falls to the bottom due to gravity. We use solubility rules to predict what will happen when two soluble salts are combined. The formation of a precipitate will drive a reaction to completion. Why? Well, why would the following not really happen. ...
... precipitate forms a , that then falls to the bottom due to gravity. We use solubility rules to predict what will happen when two soluble salts are combined. The formation of a precipitate will drive a reaction to completion. Why? Well, why would the following not really happen. ...
Class-XII, Summer assignment
... 36. Write the conditions to maximize the yield of H2SO4 by Contact process. Ans: The reaction is exothermic, reversible and the forward reaction leads to a decrease in volume. Therefore, low temperature and high pressure are the favourable conditions for maximum yield. But the temperature should not ...
... 36. Write the conditions to maximize the yield of H2SO4 by Contact process. Ans: The reaction is exothermic, reversible and the forward reaction leads to a decrease in volume. Therefore, low temperature and high pressure are the favourable conditions for maximum yield. But the temperature should not ...
Li K-edge XANES and Li(1s) XPS Spectra of Lithium Compounds
... Lithium reacts slowly with water to form a colorless solution of lithium hydroxide and molecular hydrogen and vigorously with all halogens to form halides. In four lithium halides, lithium fluoride is insoluble in aqueous solution like ones of magnesium and calcium. So some properties of lithium res ...
... Lithium reacts slowly with water to form a colorless solution of lithium hydroxide and molecular hydrogen and vigorously with all halogens to form halides. In four lithium halides, lithium fluoride is insoluble in aqueous solution like ones of magnesium and calcium. So some properties of lithium res ...
PIB - Unit 6 - Chemical Reactions - Student
... The substances that undergo a chemical reaction are the reactants. The new substances formed are the products. Special symbols are written after formulas in equations to show a substance’s state. The designations for solid, liquid, or gas, are (s), (l), and (g), respectively. A substance dissolv ...
... The substances that undergo a chemical reaction are the reactants. The new substances formed are the products. Special symbols are written after formulas in equations to show a substance’s state. The designations for solid, liquid, or gas, are (s), (l), and (g), respectively. A substance dissolv ...
Solids Chemistry XII - The Gurukul Institute
... f) Vacancies are introduced in an ionic solid when a solid of higher valence is added as an impurity in it. g) Zinc oxide on heating becomes yellow. h) The cation vacancies in certain crystals make them good catalysts. i) Non- Stokchieometric sodium chloride is a yellow solid. j) Solids with F – cen ...
... f) Vacancies are introduced in an ionic solid when a solid of higher valence is added as an impurity in it. g) Zinc oxide on heating becomes yellow. h) The cation vacancies in certain crystals make them good catalysts. i) Non- Stokchieometric sodium chloride is a yellow solid. j) Solids with F – cen ...
File
... – write down the charge on each particle, the mass of each particle, and where they are found in the atom. Electron = neg. charge found outside nucleus, almost zero mass Protons = pos. charge, in nucleus, 1 amu Neutron = no charge, in nucleus, 1 amu ...
... – write down the charge on each particle, the mass of each particle, and where they are found in the atom. Electron = neg. charge found outside nucleus, almost zero mass Protons = pos. charge, in nucleus, 1 amu Neutron = no charge, in nucleus, 1 amu ...
Mechanochemistry: the varied applications of mechanical bond
... claims and misinterpretations with regard to the term ‘‘mechanochemistry’’ in the literature have been tempting uncritical authors to increase the ball speed beyond an optimal level with so-called high-speed mills21 or with planetary mills also for the molecular solid-state reactions. However, high ...
... claims and misinterpretations with regard to the term ‘‘mechanochemistry’’ in the literature have been tempting uncritical authors to increase the ball speed beyond an optimal level with so-called high-speed mills21 or with planetary mills also for the molecular solid-state reactions. However, high ...
Chapters 1 to 5 - Lakeland Regional High School
... ____ 35. The main energy level that can hold only two electrons is the a. first. c. third. b. second. d. fourth. ____ 36. "Orbitals of equal energy are each occupied by one electron before any is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin" i ...
... ____ 35. The main energy level that can hold only two electrons is the a. first. c. third. b. second. d. fourth. ____ 36. "Orbitals of equal energy are each occupied by one electron before any is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin" i ...
2 CHEMICAL ARITHMATICS W MODULE - 1
... In your previous classes, you have studied how to write chemical formula of a sustance. For example, water is represented by H2O, carbon dioxide is represented by CO2, methane is represented by CH4, dinitrogen penta oxide is represented by N2O5, and so on. You are aware, formula for a molecule uses ...
... In your previous classes, you have studied how to write chemical formula of a sustance. For example, water is represented by H2O, carbon dioxide is represented by CO2, methane is represented by CH4, dinitrogen penta oxide is represented by N2O5, and so on. You are aware, formula for a molecule uses ...
Topic 3 MOLE Avodagro`s number = 6.02 x 1023 things = 1 mole 1
... a) How many grams of O2 are needed to burn 1.50 mole of octane? 1.5 mole octane (25mole O2 /2mole octane) (32 g /mole O2) = 600 g 12. One of the steps in the commercial process for converting ammonia to nitric acid involves the conversion of N H3 to NO: 4NH3 (g) + 5 O2 (g) 4NO (g) + 6 H2O (g) In a ...
... a) How many grams of O2 are needed to burn 1.50 mole of octane? 1.5 mole octane (25mole O2 /2mole octane) (32 g /mole O2) = 600 g 12. One of the steps in the commercial process for converting ammonia to nitric acid involves the conversion of N H3 to NO: 4NH3 (g) + 5 O2 (g) 4NO (g) + 6 H2O (g) In a ...
File - Ms. Puetz` science site
... To keep track of the huge numbers of atoms and molecules in samples that are large enough to see, chemists have established a unit of counting called the mole (abbreviated mol) and a unit of measure called the molar mass, which has units of g/mol. By using the idea of a mole and molar mass, you will ...
... To keep track of the huge numbers of atoms and molecules in samples that are large enough to see, chemists have established a unit of counting called the mole (abbreviated mol) and a unit of measure called the molar mass, which has units of g/mol. By using the idea of a mole and molar mass, you will ...
Presentation
... protons, 6 neutrons, and 6 electrons. • You could build an oxygen atom using 8 protons, 9 neutrons, and 8 electrons. • You could even build a gold atom with 79 protons, 118 neutrons, and 79 electrons! • As you can see, an atom does not have to have equal numbers of protons and neutrons. © Fall 2005, ...
... protons, 6 neutrons, and 6 electrons. • You could build an oxygen atom using 8 protons, 9 neutrons, and 8 electrons. • You could even build a gold atom with 79 protons, 118 neutrons, and 79 electrons! • As you can see, an atom does not have to have equal numbers of protons and neutrons. © Fall 2005, ...
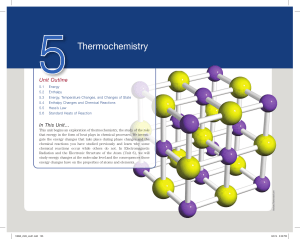
Thermochemistry
... • Chemical energy - The energy stored within the structural units of chemical substances; its quantity is determined by the type and arrangement of constituent atoms. – When substances participate in chemical reactions, chemical energy is released, stored, or converted to other forms of energy. – C ...
... • Chemical energy - The energy stored within the structural units of chemical substances; its quantity is determined by the type and arrangement of constituent atoms. – When substances participate in chemical reactions, chemical energy is released, stored, or converted to other forms of energy. – C ...
Preview Sample 2
... A. Main-group elements are also known as representative elements. B. Main-group elements are in groups labeled with the letter A. C. Main-group elements are in groups labeled with the letter B. D. Main-group elements include metals. E. Main-group elements include nonmetals. ...
... A. Main-group elements are also known as representative elements. B. Main-group elements are in groups labeled with the letter A. C. Main-group elements are in groups labeled with the letter B. D. Main-group elements include metals. E. Main-group elements include nonmetals. ...
4.2 Structure of the Nuclear Atom
... (1868–1953) carried out experiments to find the quantity of an electron’s charge. • Using this charge and Thomson’s chargeto-mass ratio of an electron, Millikan calculated an electron’s mass. • Millikan’s values for electron charge and mass are similar to those accepted today. ...
... (1868–1953) carried out experiments to find the quantity of an electron’s charge. • Using this charge and Thomson’s chargeto-mass ratio of an electron, Millikan calculated an electron’s mass. • Millikan’s values for electron charge and mass are similar to those accepted today. ...
Stoichiometry
... 6. Compound X2Y is 60% X by mass. Calculate the percent Y by mass of the compound XY3? If you have 100 g of X2Y there would be 60g of X and 40g of Y For XY3 since it has only one X atom you can think of that as ½(60g) or 30 g of X and since there’s three Y atoms you can think of that as 3(40g) or 12 ...
... 6. Compound X2Y is 60% X by mass. Calculate the percent Y by mass of the compound XY3? If you have 100 g of X2Y there would be 60g of X and 40g of Y For XY3 since it has only one X atom you can think of that as ½(60g) or 30 g of X and since there’s three Y atoms you can think of that as 3(40g) or 12 ...
m5zn_1ed95c16cede0b1
... a- what mass of NaOH will react completely with 2.4 g AlCl3? b- what mass of Al(OH)3 will form from the complete reaction of 11 g AlCl3 with enough NaOH? 4- A reaction gave practically 4.4 g while expected to give 5.00 g theoretically. Calculate the percentage yield. ...
... a- what mass of NaOH will react completely with 2.4 g AlCl3? b- what mass of Al(OH)3 will form from the complete reaction of 11 g AlCl3 with enough NaOH? 4- A reaction gave practically 4.4 g while expected to give 5.00 g theoretically. Calculate the percentage yield. ...
Chapter 5
... values are reported in units of J/g ºC or J/g K. The values do not change with the different units because a 1-degree increment is the same on both temperature scales. Notice in Table 5.3.1 that some materials, such as metals, have low specific heat capacities, which means it takes relatively li ...
... values are reported in units of J/g ºC or J/g K. The values do not change with the different units because a 1-degree increment is the same on both temperature scales. Notice in Table 5.3.1 that some materials, such as metals, have low specific heat capacities, which means it takes relatively li ...
Practice Exam I FR Answers and Explanations
... 6. Laboratory Question—9 points total (a) Steps necessary to carry out the experiment. When writing steps in an experiment, a brief list of the essential steps are a must. Steps for this experiment might include: ...
... 6. Laboratory Question—9 points total (a) Steps necessary to carry out the experiment. When writing steps in an experiment, a brief list of the essential steps are a must. Steps for this experiment might include: ...