laman web smk raja perempuan, ipoh
... 5. calcualte enthalphy changes from experimental results 6. state Hess’ law and its use to find enthalphy changes that cannot be determined directly, eg an enthalphy change of formation from enthalphy changes of combustion. 7. construct energy level diagrams relating the enthalphy to reaction path a ...
... 5. calcualte enthalphy changes from experimental results 6. state Hess’ law and its use to find enthalphy changes that cannot be determined directly, eg an enthalphy change of formation from enthalphy changes of combustion. 7. construct energy level diagrams relating the enthalphy to reaction path a ...
Chapter 6 Table of Contents
... How many laser printers did the administration have to buy? It is rather simple to show that 26 laser printers are needed for all the employees. However, what if a chemist was calculating quantities for a chemical reaction? Interestingly enough, similar calculations can be performed for chemicals as ...
... How many laser printers did the administration have to buy? It is rather simple to show that 26 laser printers are needed for all the employees. However, what if a chemist was calculating quantities for a chemical reaction? Interestingly enough, similar calculations can be performed for chemicals as ...
Mechanistic Details of the Oscillatory Belousov
... The cerium ion catalyzed oxidation of malonic acid (MA) by BrO,-, the classic Belousov-Zhabotinskiil (BZ) reaction, is the most thoroughly studied oscillating chemical reactionZ in both batch and continuous-flow, stirred tank reactor (CSTR) modes. Its basic mechanism was elucidated in 1972 by Field, ...
... The cerium ion catalyzed oxidation of malonic acid (MA) by BrO,-, the classic Belousov-Zhabotinskiil (BZ) reaction, is the most thoroughly studied oscillating chemical reactionZ in both batch and continuous-flow, stirred tank reactor (CSTR) modes. Its basic mechanism was elucidated in 1972 by Field, ...
chemistry-with-masteringchemistry-6th-edition-mcmurry-test-bank
... 15) The existence of electrons in atoms of all elements was demonstrated by A) Millikan's oil drop experiment. B) Rutherford's gold foil experiment. C) Thomson's cathode ray tube experiment. D) None of these Answer: C Topic: Section 2.3 Atomic Structure: Electrons 16) The charge-to-mass ratio of an ...
... 15) The existence of electrons in atoms of all elements was demonstrated by A) Millikan's oil drop experiment. B) Rutherford's gold foil experiment. C) Thomson's cathode ray tube experiment. D) None of these Answer: C Topic: Section 2.3 Atomic Structure: Electrons 16) The charge-to-mass ratio of an ...
CHAPTER
... In Chapter 11, you learned that volumes of gases always combine in definite ratios. This observation, called the law of combining volumes, is based on measurements of the gas volumes. When Avogadro suggested that gases combine in fixed ratios because equal volumes of gases at the same temperature an ...
... In Chapter 11, you learned that volumes of gases always combine in definite ratios. This observation, called the law of combining volumes, is based on measurements of the gas volumes. When Avogadro suggested that gases combine in fixed ratios because equal volumes of gases at the same temperature an ...
Introduction to Inorganic Chemistry
... section (2.1). Of the remainder, the majority occur in relatively concentrated deposits, from which they or their compounds can be extracted fairly easily (e.g. hydrocarbons from petroleum). For copper, lead, zinc, nickel, and tin, demand is such that less concentrated deposits have to be worked, do ...
... section (2.1). Of the remainder, the majority occur in relatively concentrated deposits, from which they or their compounds can be extracted fairly easily (e.g. hydrocarbons from petroleum). For copper, lead, zinc, nickel, and tin, demand is such that less concentrated deposits have to be worked, do ...
Chemical Reactions and Solution Stoichiometry
... Notice that in a combination reaction, the number of reactants is greater than the number of products (Figure 4.1.2). In a typical decomposition reaction (Interactive Figure 4.1.3), the number of products is greater than the number of reactants. The reaction is essentially the reverse of a combinati ...
... Notice that in a combination reaction, the number of reactants is greater than the number of products (Figure 4.1.2). In a typical decomposition reaction (Interactive Figure 4.1.3), the number of products is greater than the number of reactants. The reaction is essentially the reverse of a combinati ...
Atomic Polar Tensor Transferabllity and Atomic Charges kr the
... in ref 1. (RtY)represents the center of charge of the h brid orbital (pv),where p and v indicate orbitals of atom A, and R,,YB represents the bonding center of charge since p and v belong to different atoms, A and B, whether chemically bonded or not. These contributions in expression 1 are known, re ...
... in ref 1. (RtY)represents the center of charge of the h brid orbital (pv),where p and v indicate orbitals of atom A, and R,,YB represents the bonding center of charge since p and v belong to different atoms, A and B, whether chemically bonded or not. These contributions in expression 1 are known, re ...
expected output
... Lectures and Tutorials SYLLABUS: Quadratic functions and equations. Surds, logarithms and indices. Permutations and combinations. Series; finite, infinite, arithmetic, geometric and binomial(positive integral index only)including applications to compound interest, approximartions, growth and decay. ...
... Lectures and Tutorials SYLLABUS: Quadratic functions and equations. Surds, logarithms and indices. Permutations and combinations. Series; finite, infinite, arithmetic, geometric and binomial(positive integral index only)including applications to compound interest, approximartions, growth and decay. ...
expected output
... information on trends, global and local distribution; Justification of importance of course Biology of HIV/AIDS: Overview of immune system, natural immunity to HIV/AIDS; the AIDS Virus and its life Cycle, disease progression (epidemiology), transmission and diagnosis. Treatment and Management: Nutri ...
... information on trends, global and local distribution; Justification of importance of course Biology of HIV/AIDS: Overview of immune system, natural immunity to HIV/AIDS; the AIDS Virus and its life Cycle, disease progression (epidemiology), transmission and diagnosis. Treatment and Management: Nutri ...
Atomic Masses
... C(s) + O2 (g) CO2 (g) of carbon 1 Atom reacts with 1 Molecule to yield 1 Molecule • But individual atoms are to small to see • We can easily count things like jelly beans and pennies, but atoms are far too small to be counted ...
... C(s) + O2 (g) CO2 (g) of carbon 1 Atom reacts with 1 Molecule to yield 1 Molecule • But individual atoms are to small to see • We can easily count things like jelly beans and pennies, but atoms are far too small to be counted ...
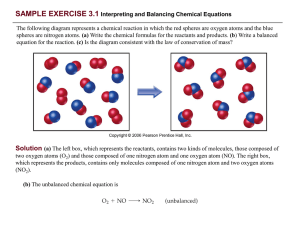
PRACTICE EXERCISE - Needham.K12.ma.us
... SAMPLE EXERCISE 3.4 Writing Balanced Equations for Combustion Reactions Write the balanced equation for the reaction that occurs when methanol, CH3OH(l), is burned in air. ...
... SAMPLE EXERCISE 3.4 Writing Balanced Equations for Combustion Reactions Write the balanced equation for the reaction that occurs when methanol, CH3OH(l), is burned in air. ...
CB document - mvhs
... states that the energy of the universe is constant and while energy can be converted from one form into another, it cannot be created or destroyed. Considering this, energy exchanged between a system and its surroundings can be considered to offset one another—the same amount of energy leaving a sys ...
... states that the energy of the universe is constant and while energy can be converted from one form into another, it cannot be created or destroyed. Considering this, energy exchanged between a system and its surroundings can be considered to offset one another—the same amount of energy leaving a sys ...
2014_S4_CHM_NORMAL (ALL)
... 53. Element X (atomic number 11) reacts with element Y (atomic number 16) to form an ionic compound. Each atom of X loses one electron and each atom of Y accepts two electrons to form a compound with formula X2Y. 54. Consider the following information: ...
... 53. Element X (atomic number 11) reacts with element Y (atomic number 16) to form an ionic compound. Each atom of X loses one electron and each atom of Y accepts two electrons to form a compound with formula X2Y. 54. Consider the following information: ...
Exam 2 Key
... 6. How does the kinetic-molecular theory of gases help explain why a helium-filled balloon shrinks if it is taken outside on a cold winter day? (10 points) You should discuss how the kinetic energy of a gas depends on temperature. As T decreases, KE decreases. As KE drops, the average velocity of a ...
... 6. How does the kinetic-molecular theory of gases help explain why a helium-filled balloon shrinks if it is taken outside on a cold winter day? (10 points) You should discuss how the kinetic energy of a gas depends on temperature. As T decreases, KE decreases. As KE drops, the average velocity of a ...
Higher Chemistry Resources Guide - Glow Blogs
... Education Scotland has a page of resources on Temperature and Kinetic Energy at: http://bit.ly/1ebf3iq Details of RSC experiment using sodium thiosufate and acid to investigate the effect of temperature on reaction rate: http://rsc.li/1gxsjfQ A computer simulation of the above experiment: http://bit ...
... Education Scotland has a page of resources on Temperature and Kinetic Energy at: http://bit.ly/1ebf3iq Details of RSC experiment using sodium thiosufate and acid to investigate the effect of temperature on reaction rate: http://rsc.li/1gxsjfQ A computer simulation of the above experiment: http://bit ...
Introduction to Inorganic Chemistry
... section (2.1). Of the remainder, the majority occur in relatively concentrated deposits, from which they or their compounds can be extracted fairly easily (e.g. hydrocarbons from petroleum). For copper, lead, zinc, nickel, and tin, demand is such that less concentrated deposits have to be worked, do ...
... section (2.1). Of the remainder, the majority occur in relatively concentrated deposits, from which they or their compounds can be extracted fairly easily (e.g. hydrocarbons from petroleum). For copper, lead, zinc, nickel, and tin, demand is such that less concentrated deposits have to be worked, do ...
35 IChO Problems 1-13
... radioisotopes are alpha emitters and were created at the time of nucleosynthesis. Their decay is followed by a different sequence of alpha (4He2+) and beta (β-) disintegration's, which lead through successive transmutations of intermediate radioactive products to stable lead isotopes, 206Pb and 207P ...
... radioisotopes are alpha emitters and were created at the time of nucleosynthesis. Their decay is followed by a different sequence of alpha (4He2+) and beta (β-) disintegration's, which lead through successive transmutations of intermediate radioactive products to stable lead isotopes, 206Pb and 207P ...
- Vijay Education Academy
... contained in a bottle and told him that he would rub the bangles with this silvery liquid. Using his knowledge of chemistry, Ram immediately saw through the trick and asked his mother not to get the bangles polished and requested the man to please go away. Read this passage and answer the following ...
... contained in a bottle and told him that he would rub the bangles with this silvery liquid. Using his knowledge of chemistry, Ram immediately saw through the trick and asked his mother not to get the bangles polished and requested the man to please go away. Read this passage and answer the following ...
Higher Chemistry Resources Guide - Glow Blogs
... The following pages show the Mandatory Course key areas table from the SQA Higher Chemistry Course and Unit Support Notes. An additional fourth column has been included which contains hyperlinks to useful resources. Please note: Practitioners are not required to use the resources listed – they are o ...
... The following pages show the Mandatory Course key areas table from the SQA Higher Chemistry Course and Unit Support Notes. An additional fourth column has been included which contains hyperlinks to useful resources. Please note: Practitioners are not required to use the resources listed – they are o ...
Chem 1B Fa2015 FinalExam Review
... __ NH3(g) + __ O2(g) + __ H2O(l) __ HNO3(aq) + __ NO(g) + __ H2O(g) (a) Balance the above equation using the smallest integer coefficients. (b) If concentrated nitric acid contains 70.0% (by mass) of HNO3 and the solution has a density of 1.48 g/mL, what is the molar concentration of HNO3 in conce ...
... __ NH3(g) + __ O2(g) + __ H2O(l) __ HNO3(aq) + __ NO(g) + __ H2O(g) (a) Balance the above equation using the smallest integer coefficients. (b) If concentrated nitric acid contains 70.0% (by mass) of HNO3 and the solution has a density of 1.48 g/mL, what is the molar concentration of HNO3 in conce ...
File
... n textbook — the bombarding electrons in a mass spectrometer have a high kinetic energy n answer — the electrons are fast moving Some answers are expanded to show the logic of the calculation. For instance, the conversion of 23.7 cm3 of solution to dm3 in the answer to Question 9 of the first chap ...
... n textbook — the bombarding electrons in a mass spectrometer have a high kinetic energy n answer — the electrons are fast moving Some answers are expanded to show the logic of the calculation. For instance, the conversion of 23.7 cm3 of solution to dm3 in the answer to Question 9 of the first chap ...