Chapter 3 HWsolutions (from Handout)
... Thus, we arrive at the formula C3.339H6.55O3.331, which gives the identity and the mole ratios of atoms present. However, chemical formulas are written with whole numbers. Try to convert to whole numbers by dividing all the subscripts by the smallest subscript (3.331). C: ...
... Thus, we arrive at the formula C3.339H6.55O3.331, which gives the identity and the mole ratios of atoms present. However, chemical formulas are written with whole numbers. Try to convert to whole numbers by dividing all the subscripts by the smallest subscript (3.331). C: ...
M.Sc. Chemistry - Periyar University
... resins and silicone polymers. Functional polymers, Fire retarding polymers and electrically conducting polymers. Biomedical polymers – contact lens, dental polymers, artificial heart, kidney, skin and blood cells. ...
... resins and silicone polymers. Functional polymers, Fire retarding polymers and electrically conducting polymers. Biomedical polymers – contact lens, dental polymers, artificial heart, kidney, skin and blood cells. ...
Chemical Reactions
... A decomposition reaction is a reaction has one reactant, and two or more products. CaCO3(s) CaO(s) + CO2(g) ...
... A decomposition reaction is a reaction has one reactant, and two or more products. CaCO3(s) CaO(s) + CO2(g) ...
8 theoretical problems 2 practical problems
... For proposal B only the slow step is critical in determining the dependence of the rate constant on the temperature. The complexation step is very stable which explains the negative activation energy. For proposal B: k = A e-EB / RT = A e+83.60/RT which decreases with increasing T THE COMPETITION PR ...
... For proposal B only the slow step is critical in determining the dependence of the rate constant on the temperature. The complexation step is very stable which explains the negative activation energy. For proposal B: k = A e-EB / RT = A e+83.60/RT which decreases with increasing T THE COMPETITION PR ...
Physical chemistry and transition elements 5.1 Rates, equilibrium
... The copper half cell would have been set up so that copper metal was in contact with its ions: a strip of copper would have been placed in a solution such as CuSO4(aq), at a concentration of 1 mol dm−3 and a temperature of 298 K. The copper metal would have been the electrode and would have been con ...
... The copper half cell would have been set up so that copper metal was in contact with its ions: a strip of copper would have been placed in a solution such as CuSO4(aq), at a concentration of 1 mol dm−3 and a temperature of 298 K. The copper metal would have been the electrode and would have been con ...
document
... a. Calculate the volume of reactant solution needed to perform a reaction. b. Understand how to perform a titration. c. Calculate the quantity of substance in a titrated solution. Copyright © Cengage Learning. All rights reserved. ...
... a. Calculate the volume of reactant solution needed to perform a reaction. b. Understand how to perform a titration. c. Calculate the quantity of substance in a titrated solution. Copyright © Cengage Learning. All rights reserved. ...
Chapter 3:Mass Relationships in Chemical Reactions
... H2 (g) + Cl2 (g) HCl (g) • Notice the subscript for H and Cl is 2, therefore we have 2 atoms of each substance. In the products, we have HCl, 1 atom of each. We can balance the equation by putting a 2 in front HCl. H2 (g) + Cl2(g) 2 HCl (g) ...
... H2 (g) + Cl2 (g) HCl (g) • Notice the subscript for H and Cl is 2, therefore we have 2 atoms of each substance. In the products, we have HCl, 1 atom of each. We can balance the equation by putting a 2 in front HCl. H2 (g) + Cl2(g) 2 HCl (g) ...
406 K (English version)
... conceived: moving from a transmission method of teaching (expository teaching model) to a method of knowledge development. It involves prioritising the learner-centred approach: Increasing the number of secondary school teachers and improving their performance through ICT. Integrating ICT tools ...
... conceived: moving from a transmission method of teaching (expository teaching model) to a method of knowledge development. It involves prioritising the learner-centred approach: Increasing the number of secondary school teachers and improving their performance through ICT. Integrating ICT tools ...
1.8 M - Thierry Karsenti
... a) both of them have definite composition b) both of them do not have definite composition c) both of them have uniform composition d) both of them are formed by the chemical combination of two or more ...
... a) both of them have definite composition b) both of them do not have definite composition c) both of them have uniform composition d) both of them are formed by the chemical combination of two or more ...
Molecules, Compounds, and Chemical Equations
... 3.3 Representing Compounds: Chemical Formulas and Molecular Models The quickest and easiest way to represent a compound is with its chemical formula, which indicates the elements present in the compound and the relative number of atoms or ions of each. For example, H2O is the chemical formula for wa ...
... 3.3 Representing Compounds: Chemical Formulas and Molecular Models The quickest and easiest way to represent a compound is with its chemical formula, which indicates the elements present in the compound and the relative number of atoms or ions of each. For example, H2O is the chemical formula for wa ...
Magic of Chemical Reactions 2. - mt
... If reactants or products are present as solution in excess of water, the symbol used is ....................... . According to ......................., the total mass of reactants is equal to the total mass of products during a chemical reaction. The chemical formula of POP is ...................... ...
... If reactants or products are present as solution in excess of water, the symbol used is ....................... . According to ......................., the total mass of reactants is equal to the total mass of products during a chemical reaction. The chemical formula of POP is ...................... ...
sample chapter
... in which CH3COO⫺ is called the acetate ion. (In this book we will use the term dissociation for ionic compounds and ionization for acids and bases.) By writing the formula of acetic acid as CH3COOH we indicate that the ionizable proton is in the COOH group. The double arrow Δ in an equation means t ...
... in which CH3COO⫺ is called the acetate ion. (In this book we will use the term dissociation for ionic compounds and ionization for acids and bases.) By writing the formula of acetic acid as CH3COOH we indicate that the ionizable proton is in the COOH group. The double arrow Δ in an equation means t ...
Document
... 1mol Al 2 mol Al2O3 101.96 g Al2O3 23.4 g Al x x x 26.98 g Al 4 mol Al 1 mol Al2O3 = 44.2 g Al2O3 39.3 g of aluminum oxide are formed. What is the percentage yield? ...
... 1mol Al 2 mol Al2O3 101.96 g Al2O3 23.4 g Al x x x 26.98 g Al 4 mol Al 1 mol Al2O3 = 44.2 g Al2O3 39.3 g of aluminum oxide are formed. What is the percentage yield? ...
pH and ORP Applications Cyanide Waste Treatment
... oxidizing agent. In this application, chlorine accepts electrons from the cyanide to oxidize it, while simultaneously the chlorine is being reduced to chloride. ORP is a measure of the status of an oxidation-reduction reaction. The gold electrode detects the solution抯 ability to accept or donate ele ...
... oxidizing agent. In this application, chlorine accepts electrons from the cyanide to oxidize it, while simultaneously the chlorine is being reduced to chloride. ORP is a measure of the status of an oxidation-reduction reaction. The gold electrode detects the solution抯 ability to accept or donate ele ...
M.Sc. Part-I Chemistry - North Maharashtra University
... The internal examination of 20 Marks for practical courses will be held before the annual practical examination. A student will not be permitted to appear at the practical examination unless he / she produce a certified journal. If the journal is lost, the student should produce a certificate from H ...
... The internal examination of 20 Marks for practical courses will be held before the annual practical examination. A student will not be permitted to appear at the practical examination unless he / she produce a certified journal. If the journal is lost, the student should produce a certificate from H ...
CHEM 121 Chp 5 Spaulding
... How many grams of NH3 are produced from 8.23 g of H2? Use molar mass to convert g to moles Use molar ratio to convert between moles Use molar mass to convert moles to g ...
... How many grams of NH3 are produced from 8.23 g of H2? Use molar mass to convert g to moles Use molar ratio to convert between moles Use molar mass to convert moles to g ...
Photogeneration of Hydride Donors and Their Use Toward CO2
... Our theoretical calculations predict that free CO is difficult to convert to the formyl anion by hydride transfer reactions, however, M−CO is much easier to ΔH‡ = −0.6 kcal/mol convert to M−CHO. Our calculations also show that ΔG‡ = 12.6 kcal/mol the further photoreduction of [1•HH]2+ can create a [ ...
... Our theoretical calculations predict that free CO is difficult to convert to the formyl anion by hydride transfer reactions, however, M−CO is much easier to ΔH‡ = −0.6 kcal/mol convert to M−CHO. Our calculations also show that ΔG‡ = 12.6 kcal/mol the further photoreduction of [1•HH]2+ can create a [ ...
Document
... 1mol Al 2 mol Al2O3 101.96 g Al2O3 23.4 g Al x x x 26.98 g Al 4 mol Al 1 mol Al2O3 = 44.2 g Al2O3 39.3 g of aluminum oxide are formed. What is the percentage yield? ...
... 1mol Al 2 mol Al2O3 101.96 g Al2O3 23.4 g Al x x x 26.98 g Al 4 mol Al 1 mol Al2O3 = 44.2 g Al2O3 39.3 g of aluminum oxide are formed. What is the percentage yield? ...
Direct production of hydrogen peroxide from CO, O2, and H2O over
... method for the preparation of various amorphous alloy catalysts for versatile hydrogenation applications.11,12 All the results presented in Table 1 were obtained using an autoclave with aqueous 0.01 M sulfuric acid as the reaction medium. It is remarkable that a much higher H2O2 formation rate could ...
... method for the preparation of various amorphous alloy catalysts for versatile hydrogenation applications.11,12 All the results presented in Table 1 were obtained using an autoclave with aqueous 0.01 M sulfuric acid as the reaction medium. It is remarkable that a much higher H2O2 formation rate could ...
File
... collection of a precipitate by filtration. They discover that the amount collected is less than what was expected. They identify and list the most likely errors in the activity. Which is a systematic error? A. B. C. D. ...
... collection of a precipitate by filtration. They discover that the amount collected is less than what was expected. They identify and list the most likely errors in the activity. Which is a systematic error? A. B. C. D. ...
Chapter 14
... A) oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air. B) oxygen is a catalyst for combustion. C) oxygen is a product of combustion. D) nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature. E) nitrogen is a ...
... A) oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air. B) oxygen is a catalyst for combustion. C) oxygen is a product of combustion. D) nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature. E) nitrogen is a ...
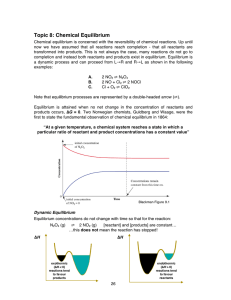
Topic 8: Chemical Equilibrium
... b) The system is in its standard state since all pressures are 1 atm -->> Q = 1 And ∆ G = ∆ Go + RTln1.00 giving ∆ G = ∆ Go = -33.3 kJ mol-1 The negative value means that in their standard states, the products have a lower free energy than the reactants so the system will move to the right. Summary ...
... b) The system is in its standard state since all pressures are 1 atm -->> Q = 1 And ∆ G = ∆ Go + RTln1.00 giving ∆ G = ∆ Go = -33.3 kJ mol-1 The negative value means that in their standard states, the products have a lower free energy than the reactants so the system will move to the right. Summary ...