June 2000 Practice Diploma
... Antibiotics formed by different species of the genus of bacteria Penicillium are among the most widely prescribed drugs in the world today. One of these antibiotics is penicillin G (benzylpenicillinic acid), which is represented as HPn(s). This acid is only slightly soluble in water. The saturated a ...
... Antibiotics formed by different species of the genus of bacteria Penicillium are among the most widely prescribed drugs in the world today. One of these antibiotics is penicillin G (benzylpenicillinic acid), which is represented as HPn(s). This acid is only slightly soluble in water. The saturated a ...
29 Sept 08 - Seattle Central
... 3. Balance the equation by inspection. Do not change the identities (formulas) of any of the reactants or products. ...
... 3. Balance the equation by inspection. Do not change the identities (formulas) of any of the reactants or products. ...
EIT Review S2012 Part 2 Dr. J. Mack CSUS Department of Chemistry
... Each element’s outermost electrons (valence) are related to the elements position on the periodic table. ...
... Each element’s outermost electrons (valence) are related to the elements position on the periodic table. ...
Instructor`s Guide to General Chemistry: Guided
... (a) The balanced reaction equation is needed to relate the number of molecules/ions of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be ...
... (a) The balanced reaction equation is needed to relate the number of molecules/ions of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be ...
chapter 16
... in a reaction mixture as a chemical reaction proceeds. The model describes the requirements that must be met before a reaction can occur, and explains why certain factors speed the reaction up or slow it down. It will help us understand why some chemical reactions are significantly reversible and wh ...
... in a reaction mixture as a chemical reaction proceeds. The model describes the requirements that must be met before a reaction can occur, and explains why certain factors speed the reaction up or slow it down. It will help us understand why some chemical reactions are significantly reversible and wh ...
Acrobat () verson
... (a) The molecular weight of aspirin is 180.16 g mol–1. Calculate the maximum mass of aspirin the student could synthesize. (b) The student collected and purified her aspirin product and wanted to calculate the yield of the reaction. Unfortunately her balance was broken, but her pH meter was in worki ...
... (a) The molecular weight of aspirin is 180.16 g mol–1. Calculate the maximum mass of aspirin the student could synthesize. (b) The student collected and purified her aspirin product and wanted to calculate the yield of the reaction. Unfortunately her balance was broken, but her pH meter was in worki ...
CHM 423 Coordination Chemistry
... Spectrochemical series (due to trend observed in crystal field spliting) and Nephelauxetic series (due to trend observed in cloud expansion). vi. to explain how complex formation can assist in stabilization of unusual oxidation states vii. to provide students with details of reaction kinetics and me ...
... Spectrochemical series (due to trend observed in crystal field spliting) and Nephelauxetic series (due to trend observed in cloud expansion). vi. to explain how complex formation can assist in stabilization of unusual oxidation states vii. to provide students with details of reaction kinetics and me ...
Chapter Three - CNG Chemistry
... at least one significant figure more than the number of significant figures in the least precisely known quantity. Doing this ensures that the precision of the calculated results is limited only by the least precisely known quantity. In part (d), this simple fact should deter you from mistakenly mul ...
... at least one significant figure more than the number of significant figures in the least precisely known quantity. Doing this ensures that the precision of the calculated results is limited only by the least precisely known quantity. In part (d), this simple fact should deter you from mistakenly mul ...
A Few Things You Might Want To Know
... density, color, reactivity, conductivity, etc.); extensive properties do (mass, ...
... density, color, reactivity, conductivity, etc.); extensive properties do (mass, ...
Chem. 1310 Fall 2005 Final Exam-white ... Name _________________________________ Section Number ___________________
... a. increase the entropy of the universe. b. decrease the energy of the universe. Answer: a 22. Which of the following are generally true? a. Intermolecular forces are weaker than covalent bonds. b. Intermolecular forces are more directional than covalent bonds. c. Any molecule in a gas experiences i ...
... a. increase the entropy of the universe. b. decrease the energy of the universe. Answer: a 22. Which of the following are generally true? a. Intermolecular forces are weaker than covalent bonds. b. Intermolecular forces are more directional than covalent bonds. c. Any molecule in a gas experiences i ...
here
... their atoms. Since those atoms are indivisible, they obviously cannot be destroyed, so the total number of atoms in the entire system must stay the same. If the total number of atoms stays the same, the total amount of matter stays the same, and therefore, the mass stays the same. Now let’s consider ...
... their atoms. Since those atoms are indivisible, they obviously cannot be destroyed, so the total number of atoms in the entire system must stay the same. If the total number of atoms stays the same, the total amount of matter stays the same, and therefore, the mass stays the same. Now let’s consider ...
Modern inorganic chemistry
... Holliday. This new book, like its predecessor, should also be of value in first-year tertiary level chemistry courses. The new syllabuses have made it possible to go much further in systematising and explaining the facts of inorganic chemistry, and in this book the first four chapters—-the periodic ...
... Holliday. This new book, like its predecessor, should also be of value in first-year tertiary level chemistry courses. The new syllabuses have made it possible to go much further in systematising and explaining the facts of inorganic chemistry, and in this book the first four chapters—-the periodic ...
Question Bank - Edudel.nic.in
... You are provided with three test tubes A, B and C. A contains distilled water, B and C contains acidic and basic solutions respectively. If you are given only blue litmus paper, how will you identify the nature of solutions in three tubes. ...
... You are provided with three test tubes A, B and C. A contains distilled water, B and C contains acidic and basic solutions respectively. If you are given only blue litmus paper, how will you identify the nature of solutions in three tubes. ...
Chapter 09 An Overview of Chemical Reactions Notes
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
Answers - logo Pre-U Chemistry Textbook
... which leads to a bond angle of 120°. The nitrogen in ammonia has three bonding pairs and one lone pair of electrons. The repulsion is greatest between the lone pair and the bonding pairs. This leads to a squashing of the tetrahedral angle leading to a pyramidal shape with an HNH bond angle of 107°. ...
... which leads to a bond angle of 120°. The nitrogen in ammonia has three bonding pairs and one lone pair of electrons. The repulsion is greatest between the lone pair and the bonding pairs. This leads to a squashing of the tetrahedral angle leading to a pyramidal shape with an HNH bond angle of 107°. ...
Chapter 03 - KFUPM Faculty List
... So when 10 g SO3 are produced, then all O2 is used up, the reaction stops and some S is left over: O2 is the limiting reactant, S the excess reactant. The other possible method (to calculate how much oxygen is needed to react with all the sulfur) gives the same result: ...
... So when 10 g SO3 are produced, then all O2 is used up, the reaction stops and some S is left over: O2 is the limiting reactant, S the excess reactant. The other possible method (to calculate how much oxygen is needed to react with all the sulfur) gives the same result: ...
- Catalyst
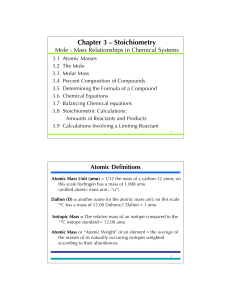
... Atomic Definitions Atomic Mass Unit (amu) = 1/12 the mass of a carbon-12 atom; on this scale hydrogen has a mass of 1.008 amu (unified atomic mass unit...“u”) Dalton (D) = another name for the atomic mass unit; on this scale 12C has a mass of 12.00 Daltons;1 Dalton = 1 amu Isotopic Mass = The relati ...
... Atomic Definitions Atomic Mass Unit (amu) = 1/12 the mass of a carbon-12 atom; on this scale hydrogen has a mass of 1.008 amu (unified atomic mass unit...“u”) Dalton (D) = another name for the atomic mass unit; on this scale 12C has a mass of 12.00 Daltons;1 Dalton = 1 amu Isotopic Mass = The relati ...
Ch 3
... In a lifetime, the average American uses 1750lb(794g) of copper in coins, plumbing, and wiring. Copper is obtained from sulfide ores, such as chalcocite, or copper(I) sulfide, by a multistage process. After an initial grinding step, the first stage is to “roast” the ore (heat it strongly with oxygen ...
... In a lifetime, the average American uses 1750lb(794g) of copper in coins, plumbing, and wiring. Copper is obtained from sulfide ores, such as chalcocite, or copper(I) sulfide, by a multistage process. After an initial grinding step, the first stage is to “roast” the ore (heat it strongly with oxygen ...
Role of Chemical Reaction Engineering in Sustainable
... In this way, n-butane and oxygen are not in direct contact and this leads to minimizing side reactions and higher maleic anhydride selectivity (up to 90 %) is therefore obtained4,6. Figure 4 shows the circulating fluid bed reactor configuration. Again, to enable the use of CFB extensive catalyst dev ...
... In this way, n-butane and oxygen are not in direct contact and this leads to minimizing side reactions and higher maleic anhydride selectivity (up to 90 %) is therefore obtained4,6. Figure 4 shows the circulating fluid bed reactor configuration. Again, to enable the use of CFB extensive catalyst dev ...
chemistry-subject test5 w. solutions
... The following Subject Test and explanations contains questions not in test format and should be used to practice and to assess your mastery of the foundation content necessary for success on the MCAT. Simply memorizing facts is not sufficient to achieve high scores; however, an incomplete understand ...
... The following Subject Test and explanations contains questions not in test format and should be used to practice and to assess your mastery of the foundation content necessary for success on the MCAT. Simply memorizing facts is not sufficient to achieve high scores; however, an incomplete understand ...
BSC with Chemistry CBCS Syllabus 2016-17
... Uncertainty principle. Hydrogen atom spectra. Need of a new approach to Atomic structure. Schrodinger wave equation and meaning of various terms in it. Significance of ψ and ψ 2. Radial and angular nodes and their significance. Radial distribution functions and the concept of the most probable dista ...
... Uncertainty principle. Hydrogen atom spectra. Need of a new approach to Atomic structure. Schrodinger wave equation and meaning of various terms in it. Significance of ψ and ψ 2. Radial and angular nodes and their significance. Radial distribution functions and the concept of the most probable dista ...