* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Presentation
Survey
Document related concepts
Transcript
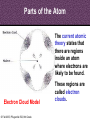
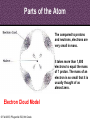
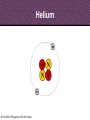
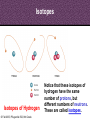
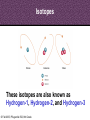
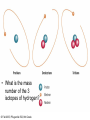
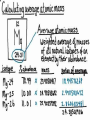
8th Grade Science Alabama Course of Study • #2 Describe the structure of atoms, including the location of protons, neutrons, and electrons. – Identifying the charge of each subatomic particle – Identifying Democritus and Dalton as contributors to the atomic theory © Fall 2005, Pflugerville ISD, 8th Grade Chapter 2- Introduction to Atoms Section 1: Development of the Atomic Theory Section 2: The Atom © Fall 2005, Pflugerville ISD, 8th Grade Outcomes • Students will: – Describe some of the experiments that led to the current atomic theory. – Compare the different models of the atom. – Explain how the atomic theory has changed as scientists have discovered new information about the atom. © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory What Do You Think? Imagine that you have cut a penny in half. Then, you take one piece and half it again. Will this continue forever, or will you come to a point where no more cutting is possible? © Fall 2005, Pflugerville ISD, 8th Grade • Remember, matter what everything is made of. • The smallest part of matter are called atoms. • All matter is made of atoms. • It started with a man, a knife, and an apple. © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Democritus was a Greek philosopher who theorized that all matter was made of invisible particles called atoms. Democritus of Abdera, about 460-370 BCE © Fall 2005, Pflugerville ISD, 8th Grade The word atom means uncuttable or indivisible. Development of the Atomic Theory His views contrasted those of Aristotle, who believed in the four elements; earth, water, air, fire. © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Most of our knowledge of Democritus comes from negative remarks about him in others’ writings. © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory John Dalton 1766-1844 © Fall 2005, Pflugerville ISD, 8th Grade Dalton, a British chemist and teacher, did studies and experiments in weather, colorblindness, and gases. Development of the Atomic Theory © Fall 2005, Pflugerville ISD, 8th Grade He noticed that elements, a substance that cannot be separated or broken down into simpler substances by chemical means, combine in specific proportions to form compounds, a substance made up of atoms of two or more different elements joined by chemical bonds,, and theorized that their atoms combine at the same proportions Development of the Atomic Theory Thomson’s experiments using a cathode-ray tube showed that smaller particles make up atoms Joseph John “J.J.” Thomson 1856-1940 © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Thomson received the Nobel Prize in physics in 1906 for his discovery of the electron. © Fall 2005, Pflugerville ISD, 8th Grade Plum-pudding Model • Thomson proposed that electrons were located throughout an atom like plums in a pudding, as shown in this model. • Today, you might call Thomson’s model the chocolate chip icecream model. © Fall 2005, Pflugerville ISD, 8th Grade Bell Ringer • Prediction: • What do you think was wrong with the plumpudding model? Explain. © Fall 2005, Pflugerville ISD, 8th Grade VIDEO • Jot Notes as you watch the video • • © Fall 2005, Pflugerville ISD, 8th Grade Duell Chemistry Gold Foil Experiment Development of the Atomic Theory Rutherford, a former student of Thomson’s from New Zealand, tested his teacher’s theories in his Gold Foil Experiment. Ernest Rutherford 1871- 1937 © Fall 2005, Pflugerville ISD, 8th Grade •He expected his alpha particles to go straight through the foil, and most of them did. Development of the Atomic Theory •But some of the particles were deflected or bounced straight back! Rutherford’s Gold Foil Experiment © Fall 2005, Pflugerville ISD, 8th Grade •This showed that a nucleus with a positive charge makes up the center of an atom. Rutherford’s Model of the Atom • Rutherford’s model of the atom had electrons surrounding the nucleus at a distance. • He calculated that the diameter of the nucleus was 100,000 times smaller than the diameter of the gold atom. Similar to a stick pin on a football field. © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Bohr, a Danish scientist who worked with Rutherford, described the motion of electrons around the nucleus. Niels Bohr 1885-1962 © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Bohr’s Atomic Model © Fall 2005, Pflugerville ISD, 8th Grade Bohr said that electrons orbit the nucleus at specific energy levels, and can move from one level to another. Development of the Atomic Theory To do this, Bohr said, the electrons must absorb or release energy, often in the form of light. © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory Erwin Schrödinger and Werner Heisenberg’s work with the uncertainty principle explained that electrons do not travel in orbits. In fact, the exact path of a moving electron Schrödinger & Heisenberg cannot be predicted. © Fall 2005, Pflugerville ISD, 8th Grade Development of the Atomic Theory The current atomic theory states that there are regions inside an atom where electrons are likely to be found. Electron Cloud Model © Fall 2005, Pflugerville ISD, 8th Grade These regions are called electron clouds. Summary • Democritus thought that matter is composed of atoms. • Dalton based his theory on observations of how elements combine. • Thomson discovered electrons in atoms. • Rutherford discovered that atoms are mostly empty space with a dense, positive nucleus. • Bohr proposed that electrons are located in levels at certain distances from the nucleus. • The electron-cloud model represents the current atomic theory. © Fall 2005, Pflugerville ISD, 8th Grade Quiz • What error did Thomson find in Dalton’s atomic theory? • What is the name for Thomson’s model of the atom • What is the current model of the atom called? © Fall 2005, Pflugerville ISD, 8th Grade Quiz • What error did Thomson find in Dalton’s atomic theory? • What is the name for Thomson’s model of the atom • What is the current model of the atom called? © Fall 2005, Pflugerville ISD, 8th Grade • Thomson discovered that atoms are made of smaller parts. • The plum-pudding model • The electron-cloud model Homework Create this table. Scientist Democritus Dalton Thomson Rutherford Bohr Schrodinger and Heisenberg © Fall 2005, Pflugerville ISD, 8th Grade Discovery Bell Ringer What Do You Think? Which scientist do you think made the most important discovery in regards to the atom? Explain. © Fall 2005, Pflugerville ISD, 8th Grade Turn and Talk Share your table with your neighbor. Scientist Democritus Dalton Thomson Rutherford Bohr Schrodinger and Heisenberg © Fall 2005, Pflugerville ISD, 8th Grade Discovery • Today you are going to create a book showing how the atomic theory has changed over time. • Materials: – Copy Paper (7 pieces per group) • 1 of the pages is a title page – – – – Colored Pencils/Markers/Crayons Pens or Pencils Stapler (to bind the book) Yesterday’s Notes © Fall 2005, Pflugerville ISD, 8th Grade • Steps: – 1. You will work in the groups that I assign you. – 2. You will receive two grades for today’s assignment. • 1 for participation • 1 for the following criteria – 2. You MUST include the following in your book: • Pictures of the different atomic models • The names of the scientists that we discussed – What they discovered • The dates that the discovery happened • If time permits, you may also want to include: – Drawings of the experiments – Drawings of the people – 3. Be Creative and colorful! – 4. Staple the left side of all the pages together in the correct order. © Fall 2005, Pflugerville ISD, 8th Grade The Atom What Do You Think? What is the smallest thing you have ever seen? How does it compare to the size of an atom? © Fall 2005, Pflugerville ISD, 8th Grade How Small is an Atom? • Think about a penny. • It contains about 2 X 10²² atoms. • (20,000,000,000,000,000, 000,000 atoms) • That’s 20 thousand billion billion atoms • In other words, atoms are really small! © Fall 2005, Pflugerville ISD, 8th Grade • Scientists know that aluminum is made of average-sized atoms. • An aluminum atom has a diameter of about 0.00000003 cm. That’s three onehundred-millionths of a centimeter. • Aluminum foil is about 50,000 atoms thick! © Fall 2005, Pflugerville ISD, 8th Grade Parts of the Atom Nucleus The atom is made up of even smaller parts. Protons Neutrons Electrons Because the masses of particles in atoms are so small, scientists made a new unit for them. The SI unit used to express the masses of particles in atoms is the atomic mass unit. (amu) © Fall 2005, Pflugerville ISD, 8th Grade Parts of the Atom Nucleus The nucleus is the small, dense, positively charged center of the atom. It contains most of its mass. Protons are positively charged particles in the nucleus of the atom. Neutrons are particles in the nucleus that have no charge © Fall 2005, Pflugerville ISD, 8th Grade • A neutron walks into a diner and orders a glass of orange juice at the lunch counter. When the waiter brings the juice, the neutron asks, “How much do I owe you?” • The waiter replies, “For you, no charge.” © Fall 2005, Pflugerville ISD, 8th Grade Parts of the Atom Nucleus The electrons are negatively charged particles found in the electron clouds out side the nucleus. The size of the electron cloud determine the size of the atom. © Fall 2005, Pflugerville ISD, 8th Grade Parts of the Atom The current atomic theory states that there are regions inside an atom where electrons are likely to be found. Electron Cloud Model © Fall 2005, Pflugerville ISD, 8th Grade These regions are called electron clouds. Parts of the Atom The compared to protons and neutrons, electrons are very small in mass. It takes more than 1,800 electrons to equal the mass of 1 proton. The mass of an electron is so small that it is usually thought of as almost zero. Electron Cloud Model © Fall 2005, Pflugerville ISD, 8th Grade Charges • The charges of protons and electrons are opposite but equal, so their charges cancel out. • Because an atom has no overall charge, it is neutral. • What happens if the numbers of electrons and protons are not equal? © Fall 2005, Pflugerville ISD, 8th Grade • The atom becomes a charged particle called an ion. • An atom that loses and electron becomes a positively-charged ion. • An atom that gains one or more extra electrons become a negativelycharged ion. • The following image shows Na losing an electron and Cl gaining an electron • • Thus the Na becomes Na+ The Cl becomes Cl- © Fall 2005, Pflugerville ISD, 8th Grade VIDEO • Atoms vs. Ions © Fall 2005, Pflugerville ISD, 8th Grade • How does an atom become a positively-charged ion? © Fall 2005, Pflugerville ISD, 8th Grade • How does an atom become a positively-charged ion? • An atom becomes a positively-charged ion when it loses an electron. © Fall 2005, Pflugerville ISD, 8th Grade Create a Visual • Create a visual representation of the element Aluminum changing from an Aluminum atom to an Aluminum ion. © Fall 2005, Pflugerville ISD, 8th Grade The Atom What Do You Think? What are some differences you use to tell one of your classmates from another? © Fall 2005, Pflugerville ISD, 8th Grade Brainstorm • Brainstorm: – How do you think you calculate the atomic number? – Write down your ideas. © Fall 2005, Pflugerville ISD, 8th Grade How do Atoms of Different Elements Differ? • There are more than 110 different elements. • The atoms of each of these elements are different from the atoms of all other elements. • Imagine that you could build an atom by putting together protons, neutrons, and electrons. © Fall 2005, Pflugerville ISD, 8th Grade Starting Simply • Let’s start with the simplest atom. – Protons and Electrons are found in all atoms. – The simplest atom is made of just one of each. – It’s so simple that it doesn’t even have a Neutron. – To “build” this atom, put just one proton in the center of the atom for the nucleus. Then, put one electron in the electron cloud. © Fall 2005, Pflugerville ISD, 8th Grade CONGRATULATIONS • You just built a hydrogen atom. © Fall 2005, Pflugerville ISD, 8th Grade Moving on! • Now draw an atom that has two protons. • Both of the protons are positively charged, so they repel one another. • You cannot form a nucleus with them UNLESS you add some neutrons. • For this atom 2 neutrons will do. • To have a neutral charge, your atom will also need two electrons outside the nucleus. © Fall 2005, Pflugerville ISD, 8th Grade Helium © Fall 2005, Pflugerville ISD, 8th Grade Building Bigger Atoms • You could build a carbon atom using 6 protons, 6 neutrons, and 6 electrons. • You could build an oxygen atom using 8 protons, 9 neutrons, and 8 electrons. • You could even build a gold atom with 79 protons, 118 neutrons, and 79 electrons! • As you can see, an atom does not have to have equal numbers of protons and neutrons. © Fall 2005, Pflugerville ISD, 8th Grade Turn and Talk • Why do you think the number of neutrons is not always the same as the number of protons or electrons? © Fall 2005, Pflugerville ISD, 8th Grade Atomic Number- the Number of Protons The Helium atom has two protons in its nucleus. Helium Atom © Fall 2005, Pflugerville ISD, 8th Grade •This means that it has the atomic number 2. Atomic Number- the Number of Protons How can you tell which elements these atoms represent? The number of protons in the nucleus of an atom is the atomic number of that atom. The atomic number is the same for all atoms of that element. © Fall 2005, Pflugerville ISD, 8th Grade • The story of the three bears will help us to remember how the atom changes. © Fall 2005, Pflugerville ISD, 8th Grade Baby Bear • Baby Bear is going to symbolize the atom as being just right. • By this I mean that the atom is in its original atomic form. • It hasn’t been changed at all. © Fall 2005, Pflugerville ISD, 8th Grade Papa Bear • Papa Bear is going to symbolize the atom in ion form. • If you remember the story Papa Bear’s stuff was too hot. • Ions are atoms that have a positive or negative charge. • They become this way because they either gain or lose electrons. © Fall 2005, Pflugerville ISD, 8th Grade Mama Bear • Last but not least, we have mama bear. • Goldilocks was not a fan of Mama Bear’s either because it just wasn’t right. It was the same as the others except for one little change. • Mama bear will represent Isotopes. © Fall 2005, Pflugerville ISD, 8th Grade Exit Slip • What is the atomic number of Barium? © Fall 2005, Pflugerville ISD, 8th Grade Bell Ringer • Isotopes have the same number of protons, but different number of _______________. © Fall 2005, Pflugerville ISD, 8th Grade Isotopes Isotopes of Hydrogen © Fall 2005, Pflugerville ISD, 8th Grade Notice that these isotopes of hydrogen have the same number of protons, but different numbers of neutrons. These are called isotopes. Isotopes These isotopes are also known as Hydrogen-1, Hydrogen-2, and Hydrogen-3 © Fall 2005, Pflugerville ISD, 8th Grade Isotopes • Atoms that are isotopes of each other are always the same element, because isotopes always have the same number of protons. • They have different numbers of neutrons, however, which gives them different masses. © Fall 2005, Pflugerville ISD, 8th Grade Properties of Isotopes • Each atom has a limited number of isotopes that are found in nature. • Some have special properties because they are unstable. • An unstable atom is an atom with a nucleus that will change over time. (This is called radioactive.) • They spontaneously fall apart after a certain amount of time. As they do, they give off smaller particles, as well as energy. © Fall 2005, Pflugerville ISD, 8th Grade Properties of Isotopes • However, isotopes of an element share most of the same chemical and physical properties. – For example, the most common oxygen isotope has 8 neutrons in the nucleus. Other isotopes of oxygen have 9 or 10 neutrons. • All 3 are colorless, odorless gases at room temperature • Each has the chemical property of combining with a substance as it burns • Different isotopes of an element even behave the same in chemical changes in your body. – For example, we breathe in various isotopes of oxygen. This is kind of like the different types of gases at the gas pump. We can use most all of them to run the vehicle. © Fall 2005, Pflugerville ISD, 8th Grade Isotopes Video • https://www.youtube.com/watch?v=GsJPxR6IfZ I © Fall 2005, Pflugerville ISD, 8th Grade Telling Isotopes Apart • You can identify each isotope of an element by its mass number. • The mass number is the sum of the protons and neutrons in an atom. • Electrons are not included because their mass is so small that they have very little effect on the atom’s total mass. © Fall 2005, Pflugerville ISD, 8th Grade © Fall 2005, Pflugerville ISD, 8th Grade • What is the mass number of the 3 isotopes of hydrogen? © Fall 2005, Pflugerville ISD, 8th Grade © Fall 2005, Pflugerville ISD, 8th Grade Naming Isotopes • To identify a specific isotope of an element, write the name of the element followed by a hyphen and the mass number of the isotope. • Hydrogen-1 – 1 proton and no neutrons – Mass number =1 • Hydrogen-2 – 1 proton and one neutron – Mass number=2 © Fall 2005, Pflugerville ISD, 8th Grade Naming Isotopes • The carbon isotope with a mass number of 12 is called Carbon-12. – If you know the atomic number (listed right above the element on the periodic table), then you can calculate the number of neutrons in carbon-12 by subtracting the atomic number from the mass number. – Carbon-12 • 12-6=6 © Fall 2005, Pflugerville ISD, 8th Grade Calculating the Mass of an Element • Most elements contain a mixture of two or more isotopes. – For example, all copper is composed of copper-63 atoms and copper-65 atoms. – The Atomic Mass of and element is the weighted average of the masses of all the naturally occurring isotopes of that element. (dealing with isotopes only) – A weighted average accounts for the percentages of each isotope that are present. – Copper, including the copper in the Statue of Liberty is 69% copper-63 and 31% copper-65. – Therefore, the atomic mass of copper is 63.6 amu. © Fall 2005, Pflugerville ISD, 8th Grade Let me walk you through what to do. • Copper-63 = 65% of all copper (63 X 0.65) = 43.47 amu • Copper-65 = 31% of all copper (65 X 0.31) = 20.15 amu • 43.47 + 20.15 = 63.62 amu © Fall 2005, Pflugerville ISD, 8th Grade © Fall 2005, Pflugerville ISD, 8th Grade Your Turn • Calculate the atomic mass of boron, which occurs naturally as 20% boron-10 and 80% boron-11. © Fall 2005, Pflugerville ISD, 8th Grade • Calculate the atomic mass of rubidium, which occurs naturally as 72% rubidium-85 and 28% rubidium-87. © Fall 2005, Pflugerville ISD, 8th Grade Exit Slip • Calculate the atomic mass of gallium, which occurs naturally as 60% gallium-69 and 40% gallium-71. • How many neutrons are there in gallium-69? • How many neutrons are there in gallium-71? © Fall 2005, Pflugerville ISD, 8th Grade Bell Ringer • What do you think keeps atoms together? © Fall 2005, Pflugerville ISD, 8th Grade Forces in Atoms • You have seen that atoms are made of smaller particles. But what are the forces (the pushes or pulls between objects) acting between these particles? • Four basic forces are at work everywhere, even within the atom. – – – – Gravitational force Electromagnetic force Strong force Weak force • They work together to give an atom its structure and properties. © Fall 2005, Pflugerville ISD, 8th Grade Gravitational Force © Fall 2005, Pflugerville ISD, 8th Grade Electromagnetic Force • As mentioned earlier, objects that have the same charge repel each other, while objects with opposite charge attract each other. • This is due to electromagnetic force. • Protons and electrons are attracted to each other because they have opposite charges. • The electromagnetic force holds the electrons around the nucleus. © Fall 2005, Pflugerville ISD, 8th Grade Strong Force • Protons push away from one another because of the electromagnetic force. • A nucleus containing two or more protons would fly apart if it weren’t for the strong force. • At the close distances between protons and neutrons in the nucleus, the strong force is greater that the electromagnetic force, so the nucleus stays together. © Fall 2005, Pflugerville ISD, 8th Grade Weak Force • This is important in radioactive atoms. • In certain unstable atoms, a neutron can change into a proton and an electron. • The weak force plays a key role in this change. © Fall 2005, Pflugerville ISD, 8th Grade Quiz • What is an atom’s mass number equal to? • How is the atomic mass of an element calculated? • How do isotopes differ from one another? © Fall 2005, Pflugerville ISD, 8th Grade Quiz • What is an atom’s mass number equal to? • How is the atomic mass of an element calculated? • How do isotopes differ from one another? © Fall 2005, Pflugerville ISD, 8th Grade • The total number of protons and neutrons in that atom. • By taking a weighted average of the mass numbers of the isotopes of that element • In the number of neutrons that they have