ExamView - 2002 AP Chemistry Exam.tst
... When the equation above is correctly balanced and all coefficients are reduced to lowest whole-number terms, the coefficient for H+(aq) is A) 2 B) 4 C) 6 D) 8 E) 14 50. Which of the following represents acceptable laboratory practice? A) Placing a hot object on a balance pan B) Using distilled water ...
... When the equation above is correctly balanced and all coefficients are reduced to lowest whole-number terms, the coefficient for H+(aq) is A) 2 B) 4 C) 6 D) 8 E) 14 50. Which of the following represents acceptable laboratory practice? A) Placing a hot object on a balance pan B) Using distilled water ...
1. True
... 19.1. Which of the following statements is FALSE? 1. The total amount of energy and matter in the Universe is constant. 2. Breaking chemical bonds is an endothermic process. 3. It is more efficient to use a primary energy source than a secondary energy source. 4. Entropy must be conserved in all che ...
... 19.1. Which of the following statements is FALSE? 1. The total amount of energy and matter in the Universe is constant. 2. Breaking chemical bonds is an endothermic process. 3. It is more efficient to use a primary energy source than a secondary energy source. 4. Entropy must be conserved in all che ...
CfE Advanced Higher Chemistry Unit 2: Organic
... When atoms approach each other, their separate sets of atomic orbitals merge to form a single set of molecular orbitals. Some of the molecular orbitals, known as 'bonding molecular orbitals', occupy the region between two nuclei. The attraction of positive nuclei to negative electrons occupying bond ...
... When atoms approach each other, their separate sets of atomic orbitals merge to form a single set of molecular orbitals. Some of the molecular orbitals, known as 'bonding molecular orbitals', occupy the region between two nuclei. The attraction of positive nuclei to negative electrons occupying bond ...
File
... (inert gases, alkali metals, Transition elements, Halogens) 30. Ionization energy is lowest for __________. (Inert gases, alkali metals, halogens, alkaline earth metals) 31. In the periodic table, the highest ionization energies are for __________. (Halogens, Noble gases, Alkali metals, Chalcogens) ...
... (inert gases, alkali metals, Transition elements, Halogens) 30. Ionization energy is lowest for __________. (Inert gases, alkali metals, halogens, alkaline earth metals) 31. In the periodic table, the highest ionization energies are for __________. (Halogens, Noble gases, Alkali metals, Chalcogens) ...
PDF on arxiv.org - at www.arxiv.org.
... The “chemical bond” is a central concept in molecular sciences, but there is no consensus as to what a bond actually is. Therefore, a variety of bonding models have been developed, each defining the structure of molecules in a different manner with the goal of explaining and predicting chemical prop ...
... The “chemical bond” is a central concept in molecular sciences, but there is no consensus as to what a bond actually is. Therefore, a variety of bonding models have been developed, each defining the structure of molecules in a different manner with the goal of explaining and predicting chemical prop ...
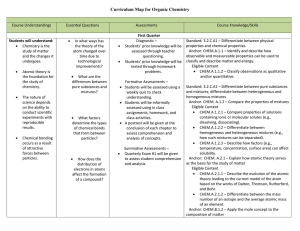
Organic Chemistry Curriculum Map - Belle Vernon Area School District
... ionization energy, electro-negativity, atomic size, and classification of elements. Anchor: CHEM.A.2.1 – Explain how atomic theory serves as the basis for the study of matter. Eligible Content CHEM.A.2.1.2 – Differentiate between the mass number of an isotope and the average atomic mass of an elem ...
... ionization energy, electro-negativity, atomic size, and classification of elements. Anchor: CHEM.A.2.1 – Explain how atomic theory serves as the basis for the study of matter. Eligible Content CHEM.A.2.1.2 – Differentiate between the mass number of an isotope and the average atomic mass of an elem ...
Physical Chemistry 3: — Chemical Kinetics
... The scriptum gives a summary of the material covered in the scheduled lectures to allow students to repeat the material more economically. It covers basic material that all chemistry students should learn irrespective of their possible inclination towards inorganic, organic or physical chemistry, bu ...
... The scriptum gives a summary of the material covered in the scheduled lectures to allow students to repeat the material more economically. It covers basic material that all chemistry students should learn irrespective of their possible inclination towards inorganic, organic or physical chemistry, bu ...
Solution - HCC Learning Web
... present in solution. Plan We write the chemical formulas of the reactants and products and then determine which product is insoluble. We then write and balance the molecular equation. Next, we write each soluble strong electrolyte as separated ions to obtain the complete ionic equation. Finally, we ...
... present in solution. Plan We write the chemical formulas of the reactants and products and then determine which product is insoluble. We then write and balance the molecular equation. Next, we write each soluble strong electrolyte as separated ions to obtain the complete ionic equation. Finally, we ...
Stoichiometry: Calculations with Chemical Formulas and
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
Second Year - WordPress.com
... The ionization energy of nitrogen is more than oxygen because of _______ a) More attraction of electrons by the nucleus b) More penetration effect c) The extra stability of half filled p – orbital d) The size of nitrogen atom is smaller. ...
... The ionization energy of nitrogen is more than oxygen because of _______ a) More attraction of electrons by the nucleus b) More penetration effect c) The extra stability of half filled p – orbital d) The size of nitrogen atom is smaller. ...
Answers - University of Waterloo
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
Chapter 3 Molecules, Compounds, and Chemical Equations How
... • Ionic compounds are composed of cations (usually a metal) and anions (usually one or more nonmetals) bound together by ionic bonds. • The basic unit of an ionic compound is the formula unit, the smallest, electrically neutral collection of ions. • The ionic compound table salt, with the formula un ...
... • Ionic compounds are composed of cations (usually a metal) and anions (usually one or more nonmetals) bound together by ionic bonds. • The basic unit of an ionic compound is the formula unit, the smallest, electrically neutral collection of ions. • The ionic compound table salt, with the formula un ...
Chapter 7
... • Each corresponds to an ORBITAL — the region of space within which an electron is found. • does NOT describe the exact location of the electron. • 2 is proportional to the probability of finding an e- at a given point. Dr. S. M. Condren ...
... • Each corresponds to an ORBITAL — the region of space within which an electron is found. • does NOT describe the exact location of the electron. • 2 is proportional to the probability of finding an e- at a given point. Dr. S. M. Condren ...
CHEM12 C04 L1 LO File
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
4 - WebAssign
... Valence electrons are transferred or shared when chemical bonds form. Lewis structures of representative elements consist of the element’s symbol and one dot for each valence electron. Copyright © Houghton Mifflin Company. All rights reserved. ...
... Valence electrons are transferred or shared when chemical bonds form. Lewis structures of representative elements consist of the element’s symbol and one dot for each valence electron. Copyright © Houghton Mifflin Company. All rights reserved. ...
4.1 Defining the Atom - Miami Beach Senior High School
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
4.1: Defining the Atom - Chemistry with Mr. Saval
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
4.1 Defining the Atom
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
4.1 Defining the Atom - Pittsfield High School
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
4.1 Defining the Atom - Miami Beach Senior High School
... future promise for the creation of atomicsized electronic devices, such as circuits and ...
... future promise for the creation of atomicsized electronic devices, such as circuits and ...