DEPARTMENT OF CHEMISTRY, CFS, IIUM
... the amount of matter is called an intensive property. A characteristic that can be observed without producing new kinds of matter is called a physical property. A characteristic that depends on how a kind of matter changes suring interactions with other kinds of matter is called chemical property. M ...
... the amount of matter is called an intensive property. A characteristic that can be observed without producing new kinds of matter is called a physical property. A characteristic that depends on how a kind of matter changes suring interactions with other kinds of matter is called chemical property. M ...
Reactions in Aqueous Solution (Brown 13th-Fossum
... • Arrhenius: substances that increase the concentration of H+ when dissolved in water. • Brønsted and Lowry: proton donors. Bases – Taste bitter and have a high pH. (Turn litmus paper blue.) • Arrhenius: Increase the concentration of OH− when dissolved in water. • Brønsted and Lowry: proton acceptor ...
... • Arrhenius: substances that increase the concentration of H+ when dissolved in water. • Brønsted and Lowry: proton donors. Bases – Taste bitter and have a high pH. (Turn litmus paper blue.) • Arrhenius: Increase the concentration of OH− when dissolved in water. • Brønsted and Lowry: proton acceptor ...
chapter 9: aqueous solutions
... 2. write the formula of the compound followed by an arrow 3. balance using coefficients 4. add state symbols (state of pure substance on the left, ((s) usually), (aq) for ions on the right) Example 1: Solid Sodium carbonate dissolves in water ...
... 2. write the formula of the compound followed by an arrow 3. balance using coefficients 4. add state symbols (state of pure substance on the left, ((s) usually), (aq) for ions on the right) Example 1: Solid Sodium carbonate dissolves in water ...
Principles of Chemistry: A Molecular Approach
... • If a beryllium atom has 4 protons, then it should weigh 4 amu; but it actually weighs 9.01 amu! ...
... • If a beryllium atom has 4 protons, then it should weigh 4 amu; but it actually weighs 9.01 amu! ...
Ch 5 HEAT IN CHEMICAL REACTIONS Chemical reactions and the
... Enthalpy Diagrams: Show the enthalpy change of a reaction. The y axis is increasing enthalpy, ∆H The reactants are written on a line then an arrow points to a line with the products. ...
... Enthalpy Diagrams: Show the enthalpy change of a reaction. The y axis is increasing enthalpy, ∆H The reactants are written on a line then an arrow points to a line with the products. ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... Principles of Chemistry: A Molecular Approach, 1st Ed. ...
... Principles of Chemistry: A Molecular Approach, 1st Ed. ...
The Structure of Matter
... charges should settle to an equilibrium and then vibrate around that position, which causes the emission of the spectrum lines. [5] Jeans then goes on to state that Earnshaw!s Theorem proved that there is no position in which there will exist a stable equilibrium. If this is true, the point charges ...
... charges should settle to an equilibrium and then vibrate around that position, which causes the emission of the spectrum lines. [5] Jeans then goes on to state that Earnshaw!s Theorem proved that there is no position in which there will exist a stable equilibrium. If this is true, the point charges ...
AP Chemistry: Total Notes Review
... o Pauli exclusion principle: no two electrons in an atom can have the same values for n, l, m1, and ms ~limits the amount of electrons that can occupy an orbital to 2 o Electron configuration: describes how the electrons are distributed among the orbitals fo an atom o Hund’s Rule: the lowest energy ...
... o Pauli exclusion principle: no two electrons in an atom can have the same values for n, l, m1, and ms ~limits the amount of electrons that can occupy an orbital to 2 o Electron configuration: describes how the electrons are distributed among the orbitals fo an atom o Hund’s Rule: the lowest energy ...
Atomic Structure
... atomic structure structure of an atom chemistry - each atom consists of a very small nucleus composed of protons and neutrons which is encircled by moving electrons some of the important properties depend on, chem4kids com atoms structure - chem4kids com this tutorial introduces atomic structure in ...
... atomic structure structure of an atom chemistry - each atom consists of a very small nucleus composed of protons and neutrons which is encircled by moving electrons some of the important properties depend on, chem4kids com atoms structure - chem4kids com this tutorial introduces atomic structure in ...
support material
... Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and other properties. Atoms of different elements are different in all respects. Atom is the smallest unit that takes part in chemical combinati ...
... Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and other properties. Atoms of different elements are different in all respects. Atom is the smallest unit that takes part in chemical combinati ...
chapter 18 (moore) - Salisbury University
... Nonspontaneous below 100 °C at 1 atm!! (b) The melting of an ice cube. Spontaneous!! (at room temperature or at any temperature above 0 °C) Spontaneous and Nonspontaneous Changes Familiarity with a large number of chemical reactions has convinced chemists that spontaneous chemical reactions occur on ...
... Nonspontaneous below 100 °C at 1 atm!! (b) The melting of an ice cube. Spontaneous!! (at room temperature or at any temperature above 0 °C) Spontaneous and Nonspontaneous Changes Familiarity with a large number of chemical reactions has convinced chemists that spontaneous chemical reactions occur on ...
Effects of antioxidants for the degradation of flame
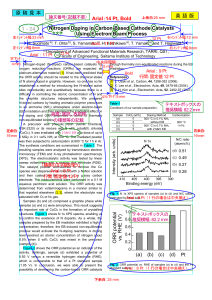
... was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electrocatalytic activity was tested by linear sweep voltamme ...
... was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electrocatalytic activity was tested by linear sweep voltamme ...
The Atom - cloudfront.net
... scientists made more exact measurements before and after reactions than ever before. In the process, they discovered some basic principles, yhich are summarized in the table' Law of Conservation of Mass Chemists measured the mass of substances before and after a reaction. The total mass of the produ ...
... scientists made more exact measurements before and after reactions than ever before. In the process, they discovered some basic principles, yhich are summarized in the table' Law of Conservation of Mass Chemists measured the mass of substances before and after a reaction. The total mass of the produ ...
Chapter 6
... • As the number of electrons increases, so does the repulsion between them. • Therefore, in atoms with more than one electron, not all orbitals on the same energy level are degenerate. • Orbital sets in the same sublevel are still degenerate. • Energy levels start to overlap in energy (e.g., 4s is l ...
... • As the number of electrons increases, so does the repulsion between them. • Therefore, in atoms with more than one electron, not all orbitals on the same energy level are degenerate. • Orbital sets in the same sublevel are still degenerate. • Energy levels start to overlap in energy (e.g., 4s is l ...
File
... trends shown in Table 19.4. Element 119 should have the smallest ionization energy, the most negative standard reduction potential, the largest radius and the smallest melting point of all the alkali metals listed in Table 19.4. It should also be radioactive like Fr. ...
... trends shown in Table 19.4. Element 119 should have the smallest ionization energy, the most negative standard reduction potential, the largest radius and the smallest melting point of all the alkali metals listed in Table 19.4. It should also be radioactive like Fr. ...
S4 Standard Grade Revision Booklet
... The extraction of a metal from it’s ore is a reduction reaction (R.I.G.). The metal ion gains electrons to form metal atoms. Iron is extracted from iron ore in a BLAST FURNACE. The 3 raw materials are, iron ore, coke and limestone. Most of the iron produced is made into steel. Reasons for Re-Cycling ...
... The extraction of a metal from it’s ore is a reduction reaction (R.I.G.). The metal ion gains electrons to form metal atoms. Iron is extracted from iron ore in a BLAST FURNACE. The 3 raw materials are, iron ore, coke and limestone. Most of the iron produced is made into steel. Reasons for Re-Cycling ...
South Pasadena • AP Chemistry
... indicates that the reaction is exothermic. Label the x-axis, y-axis, reactants, products, and what amount of activation energy is needed for each reaction. 9. What does the Ksp value indicate about an ionic solid? Ksp is a number that indicates the solubility of an ionic solid that has low to no sol ...
... indicates that the reaction is exothermic. Label the x-axis, y-axis, reactants, products, and what amount of activation energy is needed for each reaction. 9. What does the Ksp value indicate about an ionic solid? Ksp is a number that indicates the solubility of an ionic solid that has low to no sol ...
Atomic structure and the periodic tabl
... At KS3 students present the structure of the atom using Dalton’s atomic model. Dalton believed that atoms were tiny indestructible units which were solid particles and this is how it is understood by many students at KS3. At GCSE, students describe the atom as a positively charged nucleus surrounded ...
... At KS3 students present the structure of the atom using Dalton’s atomic model. Dalton believed that atoms were tiny indestructible units which were solid particles and this is how it is understood by many students at KS3. At GCSE, students describe the atom as a positively charged nucleus surrounded ...
Chemistry 400
... 8) Choose the transition (in a hydrogen atom) below that represents the absorption of the shortest wavelength photon. A) n = 1 to n = 2 B) n = 2 to n = 3 C) n = 4 to n = 5 D) n = 6 to n = 3 E) n = 3 to n = 1 9) Which of the following statements is TRUE? A) We can sometimes know the exact location an ...
... 8) Choose the transition (in a hydrogen atom) below that represents the absorption of the shortest wavelength photon. A) n = 1 to n = 2 B) n = 2 to n = 3 C) n = 4 to n = 5 D) n = 6 to n = 3 E) n = 3 to n = 1 9) Which of the following statements is TRUE? A) We can sometimes know the exact location an ...
WORD - SSS Chemistry
... ___________________________ devised the Scattering Experiment, which showed that all atoms had a small dense __________________________. ...
... ___________________________ devised the Scattering Experiment, which showed that all atoms had a small dense __________________________. ...
Part I - American Chemical Society
... hours with a current of 2.50 amps. What mass of copper metal is formed? (A) 8.88 g ...
... hours with a current of 2.50 amps. What mass of copper metal is formed? (A) 8.88 g ...