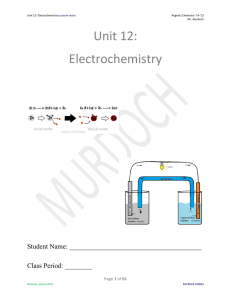
KEY Final Exam Review - Iowa State University
... Note that 16 and 3 have no common factors except 1, so both 16 and 3 had to be used to obtain the lowest common multiple of 48 for the number of electrons. 4) Add: 24H2S + 16H+ + 16NO3¯ ---> 3S8 + 16NO + 32H2O Comment: removing a factor of 8 does look tempting, doesn't it? However, the three in fron ...
... Note that 16 and 3 have no common factors except 1, so both 16 and 3 had to be used to obtain the lowest common multiple of 48 for the number of electrons. 4) Add: 24H2S + 16H+ + 16NO3¯ ---> 3S8 + 16NO + 32H2O Comment: removing a factor of 8 does look tempting, doesn't it? However, the three in fron ...
answers to part a of the national high school
... 2. Most teachers (98%) thought that this question was appropriate, and, in BC, students found it to be the easiest question on the exam, although in other provinces it was not as well done. The discrimination index was moderate. One teacher indicated that s/he did not recognise the symbol Mr (which ...
... 2. Most teachers (98%) thought that this question was appropriate, and, in BC, students found it to be the easiest question on the exam, although in other provinces it was not as well done. The discrimination index was moderate. One teacher indicated that s/he did not recognise the symbol Mr (which ...
FREE Sample Here
... http://testbankwizard.eu/Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbe ll 45) Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH3)? A) Each hydrogen atom has a partial positive charge. B) The ni ...
... http://testbankwizard.eu/Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbe ll 45) Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH3)? A) Each hydrogen atom has a partial positive charge. B) The ni ...
Chem 107 - Hughbanks Exam 1
... alphanumeric information. Print your name above, provide your UIN number, and sign the honor code statement below. ...
... alphanumeric information. Print your name above, provide your UIN number, and sign the honor code statement below. ...
Biology, 8e (Campbell) Chapter 2 The Chemical Context of Life
... A) two more protons than carbon-12. B) two more electrons than carbon-12. C) two more neutrons than carbon-12. D) A and C only E) B and C only Answer: C Topic: Concept 2.2 Skill: Knowledge/Comprehension 17) 3 H is a radioactive isotope of hydrogen. One difference between hydrogen-1 ( 11 H) and hydro ...
... A) two more protons than carbon-12. B) two more electrons than carbon-12. C) two more neutrons than carbon-12. D) A and C only E) B and C only Answer: C Topic: Concept 2.2 Skill: Knowledge/Comprehension 17) 3 H is a radioactive isotope of hydrogen. One difference between hydrogen-1 ( 11 H) and hydro ...
FREE Sample Here
... A) two more protons than carbon-12. B) two more electrons than carbon-12. C) two more neutrons than carbon-12. D) A and C only E) B and C only Answer: C Topic: Concept 2.2 Skill: Knowledge/Comprehension 17) 3 H is a radioactive isotope of hydrogen. One difference between hydrogen-1 ( 11 H) and hydro ...
... A) two more protons than carbon-12. B) two more electrons than carbon-12. C) two more neutrons than carbon-12. D) A and C only E) B and C only Answer: C Topic: Concept 2.2 Skill: Knowledge/Comprehension 17) 3 H is a radioactive isotope of hydrogen. One difference between hydrogen-1 ( 11 H) and hydro ...
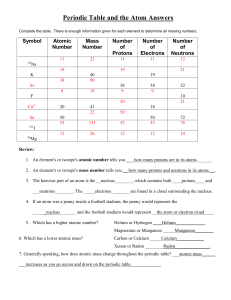
Periodic Table and the Atom Answers
... Answer: The term "pH" is defined as the negative logarithm of H+ ion concentration of a given solution; the concentration being expressed as moles per litre. Mathematically pH = - log [H+] 'pH' stands for: Power of hydrogen ion concentration, 'p' for power and 'H' for H+ ion concentration. 3) What i ...
... Answer: The term "pH" is defined as the negative logarithm of H+ ion concentration of a given solution; the concentration being expressed as moles per litre. Mathematically pH = - log [H+] 'pH' stands for: Power of hydrogen ion concentration, 'p' for power and 'H' for H+ ion concentration. 3) What i ...
Types of Radiation - Kasson
... • For example, gamma rays accompany the alpha-decay reaction of uranium-238. • The 2 in front of the γ symbol indicates that two gamma rays of different frequencies are emitted. • Because gamma rays have no effect on mass number or atomic number, it is customary to omit them from nuclear equations. ...
... • For example, gamma rays accompany the alpha-decay reaction of uranium-238. • The 2 in front of the γ symbol indicates that two gamma rays of different frequencies are emitted. • Because gamma rays have no effect on mass number or atomic number, it is customary to omit them from nuclear equations. ...
CHAPTER 2
... Octet Rule: The tendency of elements to acquire the configuration of the noble gas __________________ to them in order to have ____________ electrons in their valence shell. (Exceptions: Li, ______, and ______ acquire the configuration of ______________ and thus follow the ___________ rule.) ...
... Octet Rule: The tendency of elements to acquire the configuration of the noble gas __________________ to them in order to have ____________ electrons in their valence shell. (Exceptions: Li, ______, and ______ acquire the configuration of ______________ and thus follow the ___________ rule.) ...
SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... Gather information and discuss on :a) Group 17 elements and their physical and chemical properties. b) the similarities in chemical properties of Group 17 elements c) the relationship between chemical properties of group 17 elements with their electron arrangements Carry out experiment to investigat ...
... Gather information and discuss on :a) Group 17 elements and their physical and chemical properties. b) the similarities in chemical properties of Group 17 elements c) the relationship between chemical properties of group 17 elements with their electron arrangements Carry out experiment to investigat ...
EXAM IR - Academics
... • There is only one correct answer to each question unless otherwise noted. Any questions for which more than one response has been selected will not be counted • Your score is based solely on the number of questions you answer correctly. It is to your advantage to answer every question. • The best ...
... • There is only one correct answer to each question unless otherwise noted. Any questions for which more than one response has been selected will not be counted • Your score is based solely on the number of questions you answer correctly. It is to your advantage to answer every question. • The best ...
Welcome`to`AP`Chemistry!
... 4.) Addition)and)subtraction)using)scientific)notation)requires)that)the)two)values)to)be)combined)have)the)same)power) of)ten.))If)the)powers)of)ten)do)not)match,)the)smaller)value)should)be)converted)so)that)its)power)of)ten)is)the) same)as)that)of)the)larger)valueg)the)conversion)is)accomplished ...
... 4.) Addition)and)subtraction)using)scientific)notation)requires)that)the)two)values)to)be)combined)have)the)same)power) of)ten.))If)the)powers)of)ten)do)not)match,)the)smaller)value)should)be)converted)so)that)its)power)of)ten)is)the) same)as)that)of)the)larger)valueg)the)conversion)is)accomplished ...
Study Material - Class- XI- Chemistry
... should contain equal number of molecules. Dalton's Atomic Theory *All substances are made up of tiny, indivisible particles called atoms. *Atoms of the same element are identical in shape, size, mass and other properties. *Atoms of different elements are different in all respects. *Atom is the small ...
... should contain equal number of molecules. Dalton's Atomic Theory *All substances are made up of tiny, indivisible particles called atoms. *Atoms of the same element are identical in shape, size, mass and other properties. *Atoms of different elements are different in all respects. *Atom is the small ...
THERMOCHEMISTRY - University of the Witwatersrand
... Internal energy depends on the state or conditions of the system (e.g. pressure, temperature, location) Does not depend on how it came to be in that state. state function U only depends on Ui and Uf and not how the change occurred. e.g. if a gas sample undergoes: ...
... Internal energy depends on the state or conditions of the system (e.g. pressure, temperature, location) Does not depend on how it came to be in that state. state function U only depends on Ui and Uf and not how the change occurred. e.g. if a gas sample undergoes: ...
Chapter 3 Molecules, Compounds, and Chemical Equations
... • shorthand way of describing a reaction • provides information about the reaction formulas of reactants and products states of reactants and products relative numbers of reactant and product molecules that are required can be used to determine weights of reactants used and products that can ...
... • shorthand way of describing a reaction • provides information about the reaction formulas of reactants and products states of reactants and products relative numbers of reactant and product molecules that are required can be used to determine weights of reactants used and products that can ...
Honors Chemistry I
... 1) Be sure to identify the reactants (on the left side) and the products (on the right side) 2) Count the total number of atoms of each element on each side of the equation a. If there is an imbalance of an atom, start planning a strategy to bring balance to the equation b. You will use coefficients ...
... 1) Be sure to identify the reactants (on the left side) and the products (on the right side) 2) Count the total number of atoms of each element on each side of the equation a. If there is an imbalance of an atom, start planning a strategy to bring balance to the equation b. You will use coefficients ...
Calcium - IDC
... is limited due to the minute quantities of argon gas that are required to be measured; in practical terms the technique is useful where long geological time scales are involved (e.g. the order of 100,000 years);[5] moreover, as measurement techniques steadily improve, the utility is rising. ...
... is limited due to the minute quantities of argon gas that are required to be measured; in practical terms the technique is useful where long geological time scales are involved (e.g. the order of 100,000 years);[5] moreover, as measurement techniques steadily improve, the utility is rising. ...