Topic 15 Energetics - slider-dpchemistry-11
... (c) The first ionisation energy. Is the enthalpy change required to remove 1 mole of electrons from 1 mole of a gaseous atom, e.g. Na(g) → Na+(g) + e‾ ...
... (c) The first ionisation energy. Is the enthalpy change required to remove 1 mole of electrons from 1 mole of a gaseous atom, e.g. Na(g) → Na+(g) + e‾ ...
Theoretical problems (official version)
... Upon adding some amount of sodium thiosulphate to the solution of A the color immediately becomes red, then changes to reddish-brown, and after some minutes a darkbrown precipitate C forms (reaction 1). The solution over it is colorless. Being heated on air at 600ºC, C gives a grey powder X (reactio ...
... Upon adding some amount of sodium thiosulphate to the solution of A the color immediately becomes red, then changes to reddish-brown, and after some minutes a darkbrown precipitate C forms (reaction 1). The solution over it is colorless. Being heated on air at 600ºC, C gives a grey powder X (reactio ...
Example - Schoolwires.net
... a student heats a sample of the hydrate until all the water is gone. A 1.628 g sample of hydrate is heated to constant mass of 1.072 g. What is the value of X? ...
... a student heats a sample of the hydrate until all the water is gone. A 1.628 g sample of hydrate is heated to constant mass of 1.072 g. What is the value of X? ...
Counting Atoms
... • Your exam scores would count more heavily toward your final grade. • In this section, you will learn that the atomic mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element. ...
... • Your exam scores would count more heavily toward your final grade. • In this section, you will learn that the atomic mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element. ...
Unit 3 Physical Science: Chemical Reactions
... sulphur trioxide. Through using IUPAC nomenclature students should start to appreciate the usefulness of a common naming system. Teachers should point out to the students that molecular compounds consist of non-metals while ionic compounds consist of metals and non-metals. It should also be noted th ...
... sulphur trioxide. Through using IUPAC nomenclature students should start to appreciate the usefulness of a common naming system. Teachers should point out to the students that molecular compounds consist of non-metals while ionic compounds consist of metals and non-metals. It should also be noted th ...
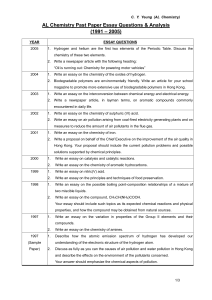
AL Chemistry Past paper essay questions
... Hong Kong. Your proposal should include the current pollution problems and possible solutions supported by chemical principles. ...
... Hong Kong. Your proposal should include the current pollution problems and possible solutions supported by chemical principles. ...
Chemistry Review 3
... a numerical setup and the calculated result. [2] 9. How do the boiling point and freezing point of a solution of water and calcium chloride at standard pressure compare to the boiling point and freezing point of water at standard pressure? 1. Both the freezing point and boiling point of the solution ...
... a numerical setup and the calculated result. [2] 9. How do the boiling point and freezing point of a solution of water and calcium chloride at standard pressure compare to the boiling point and freezing point of water at standard pressure? 1. Both the freezing point and boiling point of the solution ...
Unit 8: Reactions - Mark Rosengarten
... A chemical change where reactants are turned into products. A reaction in which one element is oxidized and another element is reduced. The gain of electron(s), causing the oxidation number of a species to become more negative. A redox reaction in which an element replaces an ion in a compound. An i ...
... A chemical change where reactants are turned into products. A reaction in which one element is oxidized and another element is reduced. The gain of electron(s), causing the oxidation number of a species to become more negative. A redox reaction in which an element replaces an ion in a compound. An i ...
Chemistry Senior External Syllabus 1998
... Candidates will be assessed by means of two examinations, and details of the papers are provided in section 8. While direct assessment of the suggested experiments and manipulative skills stated in the syllabus is not possible, they will be assessed indirectly in the examination papers. Consequently ...
... Candidates will be assessed by means of two examinations, and details of the papers are provided in section 8. While direct assessment of the suggested experiments and manipulative skills stated in the syllabus is not possible, they will be assessed indirectly in the examination papers. Consequently ...
Chapter 2 – Atoms, Ions, and the Periodic Table
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol in the fourth period of the ...
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol in the fourth period of the ...
Objective (Local, State, National – College Board)
... weak acids and bases. Arrhenius definitions are based on these observations. Bronsted Lowry theory can be introduced by considering things that are known to be acids but are not aqueous solutions. The “accounting system” of naming acids, bases conjugate acids and conjugate bases should be mastered i ...
... weak acids and bases. Arrhenius definitions are based on these observations. Bronsted Lowry theory can be introduced by considering things that are known to be acids but are not aqueous solutions. The “accounting system” of naming acids, bases conjugate acids and conjugate bases should be mastered i ...
ap chemistry 2005/2006
... identification as physical or chemical change. Lab: Determining the Specific Heat of an Unknown Metal – the specific heat capacity of a nail will be experimentally determined by measuring the temperature change of water and of the nail after it has been heated to approximately 850 degrees Celsius in ...
... identification as physical or chemical change. Lab: Determining the Specific Heat of an Unknown Metal – the specific heat capacity of a nail will be experimentally determined by measuring the temperature change of water and of the nail after it has been heated to approximately 850 degrees Celsius in ...
What are atoms?
... • In 1897, J. J. Thomson performed experiments that detected smaller particles within atoms. • In the early 1900s, Ernest Rutherford and James Chadwick revealed the nature of the dense center of an atom. • Today we have the electron cloud model. ...
... • In 1897, J. J. Thomson performed experiments that detected smaller particles within atoms. • In the early 1900s, Ernest Rutherford and James Chadwick revealed the nature of the dense center of an atom. • Today we have the electron cloud model. ...
Chapter 3 Review: Mass Relationships in Chemical Reactions Will
... Deflection: in a magnetic field, heavier isotopes get deflected less than lighter isotopes. Step 5: Detection: counts how many of each mass come through by using electric of photographic methods. ...
... Deflection: in a magnetic field, heavier isotopes get deflected less than lighter isotopes. Step 5: Detection: counts how many of each mass come through by using electric of photographic methods. ...
Chemistry Name Mr. Reger Review Guide – Ch. 9
... 10. Using the reaction below, how many grams of NaCl can be produced from 10.9 g NaOH? 3 Cl2(g) + 6 NaOH(aq) 5 NaCl(aq) + NaClO3(aq) + 3 H2O(l) 11. Use the equation in the question above to answer the following: a) What is the theoretical yield of NaClO3 if 4.0mol Cl2 is reacted with excess NaOH? ...
... 10. Using the reaction below, how many grams of NaCl can be produced from 10.9 g NaOH? 3 Cl2(g) + 6 NaOH(aq) 5 NaCl(aq) + NaClO3(aq) + 3 H2O(l) 11. Use the equation in the question above to answer the following: a) What is the theoretical yield of NaClO3 if 4.0mol Cl2 is reacted with excess NaOH? ...
Appendix N CONCENTRATION UNITS
... solutes or mixtures of solutes. Parts per million is also used to describe concentration levels to those who are unfamiliar with the concept of moles. Tap water contains a variety of dissolved impurities, mostly minerals. A simple way of describing the levels of these impurities, often called dissol ...
... solutes or mixtures of solutes. Parts per million is also used to describe concentration levels to those who are unfamiliar with the concept of moles. Tap water contains a variety of dissolved impurities, mostly minerals. A simple way of describing the levels of these impurities, often called dissol ...
幻灯片 1
... Electrons have an intrinsic rotation called spin, which may point in only two possible directions, specified by a quantum ...
... Electrons have an intrinsic rotation called spin, which may point in only two possible directions, specified by a quantum ...
The Atomic Theory Chem 111
... 2.72 Which of the following compounds are likely to be ionic? Which are likely to be molecular? Compounds of metals with nonmetals are usually ionic therefore : LiF ...
... 2.72 Which of the following compounds are likely to be ionic? Which are likely to be molecular? Compounds of metals with nonmetals are usually ionic therefore : LiF ...
Notes -- Unit 5 -- Reactions and Stoichiometry
... burns in bromine producing aluminum bromide. In a laboratory 6.0 g of aluminum reacts with excess bromine. 50.3 g of aluminum bromide are produced. What are the three types of yield. (actual, theoretical, percent) ...
... burns in bromine producing aluminum bromide. In a laboratory 6.0 g of aluminum reacts with excess bromine. 50.3 g of aluminum bromide are produced. What are the three types of yield. (actual, theoretical, percent) ...
www.xtremepapers.net
... 13 In 1999, researchers working in the USA believed that they had made a new element and that it had the following electronic structure. [Rn] 5f146d107s27p6 In which Group of the Periodic Table would you expect to find this element? A ...
... 13 In 1999, researchers working in the USA believed that they had made a new element and that it had the following electronic structure. [Rn] 5f146d107s27p6 In which Group of the Periodic Table would you expect to find this element? A ...
KEY Final Exam Review - Iowa State University
... Note that 16 and 3 have no common factors except 1, so both 16 and 3 had to be used to obtain the lowest common multiple of 48 for the number of electrons. 4) Add: 24H2S + 16H+ + 16NO3¯ ---> 3S8 + 16NO + 32H2O Comment: removing a factor of 8 does look tempting, doesn't it? However, the three in fron ...
... Note that 16 and 3 have no common factors except 1, so both 16 and 3 had to be used to obtain the lowest common multiple of 48 for the number of electrons. 4) Add: 24H2S + 16H+ + 16NO3¯ ---> 3S8 + 16NO + 32H2O Comment: removing a factor of 8 does look tempting, doesn't it? However, the three in fron ...
chemistry
... 54 State the general trend in first ionization energy for the elements in Group 2 as these elements are considered in order from top to bottom in the group. [1] 55 State, in terms of the number of electron shells, why the radius of a strontium atom in the ground state is larger than the radius of a ...
... 54 State the general trend in first ionization energy for the elements in Group 2 as these elements are considered in order from top to bottom in the group. [1] 55 State, in terms of the number of electron shells, why the radius of a strontium atom in the ground state is larger than the radius of a ...