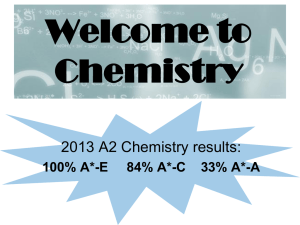
Congratulations! You have signed up for AP Chemistry for this year
... and exercises, watch the videos if necessary, and check your work. I recommend that you work them in the order in which they appear. All three assignments will probably take you about 5 hours to complete, so do not wait until the first day of school!! I expect you to have completed chapter 1 and 2 f ...
... and exercises, watch the videos if necessary, and check your work. I recommend that you work them in the order in which they appear. All three assignments will probably take you about 5 hours to complete, so do not wait until the first day of school!! I expect you to have completed chapter 1 and 2 f ...
Dr. Baxley`s Thermodynamics Worksheet
... 4. This is when a spontaneous reaction has a + ∆H and a + ∆S. The reaction is spontaneous because of its ∆S, so ∆S drives the reaction. 5. a. balance it first! ∆S is probably − because there are more gaseous reactants than products. ∆H is − because this is a combustion reaction and they are always e ...
... 4. This is when a spontaneous reaction has a + ∆H and a + ∆S. The reaction is spontaneous because of its ∆S, so ∆S drives the reaction. 5. a. balance it first! ∆S is probably − because there are more gaseous reactants than products. ∆H is − because this is a combustion reaction and they are always e ...
Matter and Measurement
... When 50.mL of 1.0M HCl and 50.mL of 1.0M NaOH are mixed in a calorimeter, the temperature of the resultant solution increases from 21.0oC to 27.5oC. Calculate the enthalpy change per mole of HCl for the reaction carried out at constant pressure, assuming that the calorimeter absorbs only a negligibl ...
... When 50.mL of 1.0M HCl and 50.mL of 1.0M NaOH are mixed in a calorimeter, the temperature of the resultant solution increases from 21.0oC to 27.5oC. Calculate the enthalpy change per mole of HCl for the reaction carried out at constant pressure, assuming that the calorimeter absorbs only a negligibl ...
Atoms – Building Blocks of Matter Notes
... Aristotle was incorrect but did not have their own theory to submit. At this time chemist did believe, based on experiments, that there were different elements and that an element was a substance that could not be broken down by chemical means. Chemist knew that some substances could transform into ...
... Aristotle was incorrect but did not have their own theory to submit. At this time chemist did believe, based on experiments, that there were different elements and that an element was a substance that could not be broken down by chemical means. Chemist knew that some substances could transform into ...
Standard - Santee Education Complex
... called the transition elements) are known for their ability to refract light as a result of their unpaired electrons. They also have several possible oxidation states. Ionic solutions of these metals are usually colored, so these metals are often used in pigments. Uranium is the last naturally occur ...
... called the transition elements) are known for their ability to refract light as a result of their unpaired electrons. They also have several possible oxidation states. Ionic solutions of these metals are usually colored, so these metals are often used in pigments. Uranium is the last naturally occur ...
redox reaction - Seattle Central College
... Earlier in the quarter we defined a solution as a homogeneous mixture; a random combination of two or more things. The part of the solution we have the most of is the solvent and the minor components of a solution are referred to as the solutes. Water is the most common solvent and a good one for io ...
... Earlier in the quarter we defined a solution as a homogeneous mixture; a random combination of two or more things. The part of the solution we have the most of is the solvent and the minor components of a solution are referred to as the solutes. Water is the most common solvent and a good one for io ...
Fall - Physical Chemistry Division
... implicated in any disease can also form amyloid fibrils, intimating that aggregation is an intrinsic property of polypeptide chains. This symposium will explore the molecular basis of protein aggregation and amyloid fibril formation from theoretical and experimental standpoints. Specific topics will ...
... implicated in any disease can also form amyloid fibrils, intimating that aggregation is an intrinsic property of polypeptide chains. This symposium will explore the molecular basis of protein aggregation and amyloid fibril formation from theoretical and experimental standpoints. Specific topics will ...
Chapter 22 - 2012 Book Archive
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
Water: The Universal Solvent
... • A student wishes to prepare 2.00 liters of 0.100-molar KIO3 (molecular weight 214 g/mol). The proper procedure is to weigh out (A) 42.8 grams of KIO3 and add 2.00 kilograms of H2O (B) 42.8 grams of KIO3 and add H2O until the final homogeneous solution has a volume of 2.00 liters (C) 21.4 grams of ...
... • A student wishes to prepare 2.00 liters of 0.100-molar KIO3 (molecular weight 214 g/mol). The proper procedure is to weigh out (A) 42.8 grams of KIO3 and add 2.00 kilograms of H2O (B) 42.8 grams of KIO3 and add H2O until the final homogeneous solution has a volume of 2.00 liters (C) 21.4 grams of ...
Computational Study of protonation of ozone
... In the course of research of ozonation was experimentally found anomalous change in pH from 6.7 to 18 by passing the ozone-oxygen mixture through distilled water [3, 4]. In discussing of the observed phenomena, it was hypothesized that the pH of distilled water is increased not only due to the forma ...
... In the course of research of ozonation was experimentally found anomalous change in pH from 6.7 to 18 by passing the ozone-oxygen mixture through distilled water [3, 4]. In discussing of the observed phenomena, it was hypothesized that the pH of distilled water is increased not only due to the forma ...
Condition - Future Website of mrbentley2
... 6. Draw the Lewis dot structures of the following ionic compounds. Then, using a different colored pen, show how one element “steals” the other’s electrons, resulting in two ions. (Hint: Some of the compounds may require multiple numbers of one type of element - be sure to draw in the extra element ...
... 6. Draw the Lewis dot structures of the following ionic compounds. Then, using a different colored pen, show how one element “steals” the other’s electrons, resulting in two ions. (Hint: Some of the compounds may require multiple numbers of one type of element - be sure to draw in the extra element ...
Chapter 2 Atoms and Elements
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws. Each element is composed of tiny, indestructible particles called atoms. All atoms of a g given element have the same mass and other properties that distinguish them from atoms of other eleme ...
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws. Each element is composed of tiny, indestructible particles called atoms. All atoms of a g given element have the same mass and other properties that distinguish them from atoms of other eleme ...
1 FORMATION OF THE ATOMIC THEORY
... atoms. Hence, there should be a limitation in the number of types of atoms. Dalton’s atomic theory requires the process in which two or more atoms combine to form matter. This is the reason why Dalton’s atom is called the “chemical atom”. (c) Proof that atoms exist When Dalton initially proposed his ...
... atoms. Hence, there should be a limitation in the number of types of atoms. Dalton’s atomic theory requires the process in which two or more atoms combine to form matter. This is the reason why Dalton’s atom is called the “chemical atom”. (c) Proof that atoms exist When Dalton initially proposed his ...
File - Science With BLT
... a. coefficients of the reactants equal the coefficients of the products. b. same number of each kind of atom appears in the reactants and in the products. c. products and reactants are the same chemicals. d. subscripts of the reactants equal the subscripts of the products. ...
... a. coefficients of the reactants equal the coefficients of the products. b. same number of each kind of atom appears in the reactants and in the products. c. products and reactants are the same chemicals. d. subscripts of the reactants equal the subscripts of the products. ...
... summarised below (see also Refs 7,8). Natta and co-workers prepared polyacetylene in 1958 by polymerising acetylene in hexane using Et3Al/Ti(OPr)4 (Et= ethyl, Pr=propyl) as a catalyst. Though the resulting material was highly crystalline and of regular structure, it was a black, air-sensitive, infus ...
Unit 8 Homework Packet
... 23. The text explains that one reason why the actual yield for a reaction may be less than the theoretical yield is side reactions. Suggest some other reasons why the percent yield for a reaction might not be 100%. ...
... 23. The text explains that one reason why the actual yield for a reaction may be less than the theoretical yield is side reactions. Suggest some other reasons why the percent yield for a reaction might not be 100%. ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
(l).
... Bohr proposed that the classical physics (Newtonian) view of matter cannot adequately explain the behavior of the electron in an atom. ...
... Bohr proposed that the classical physics (Newtonian) view of matter cannot adequately explain the behavior of the electron in an atom. ...
Section 2.6 Subatomic Particles: Protons, Neutrons and Electrons in
... Each element is made up of tiny indestructible particles called atoms. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements Atoms combine in simple whole number ratios to form compounds. Atoms of one element cannot change into atoms ...
... Each element is made up of tiny indestructible particles called atoms. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements Atoms combine in simple whole number ratios to form compounds. Atoms of one element cannot change into atoms ...
No Slide Title
... How many H atoms are in 72.5 g of C3H8O ? 1 mol C3H8O = (3 x 12) + (8 x 1) + 16 = ______ g C3H8O 1 mol C3H8O molecules = ___________ mol H atoms 1 mol H = ___________ atoms H 1 mol C3H8O 8 mol H atoms 6.022 x 1023 H atoms 72.5 g C3H8O x ...
... How many H atoms are in 72.5 g of C3H8O ? 1 mol C3H8O = (3 x 12) + (8 x 1) + 16 = ______ g C3H8O 1 mol C3H8O molecules = ___________ mol H atoms 1 mol H = ___________ atoms H 1 mol C3H8O 8 mol H atoms 6.022 x 1023 H atoms 72.5 g C3H8O x ...
FREE Sample Here
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...