Support Material
... Law of Multiple Proportions (John Dalton) : When two elements combine to form two or more compounds, then the different masses of one element, which combine with a ®xed mass of the other, bear a simple ratio to one another. Gay Lussac’s Law : When gases combine or are produced in a chemical reac ...
... Law of Multiple Proportions (John Dalton) : When two elements combine to form two or more compounds, then the different masses of one element, which combine with a ®xed mass of the other, bear a simple ratio to one another. Gay Lussac’s Law : When gases combine or are produced in a chemical reac ...
Chemistry Final Exam Review
... • Pre-periodic table patterns: Newland’s Law of Octaves, Dobereiner’s Triads • Mendeleev’s Periodic table (based on atomic mass), holes in his table • Moseley, atomic number, protons, Modern Periodic Law • basic characteristics and names of the major groups • metals, nonmetals, metalloids – “stairca ...
... • Pre-periodic table patterns: Newland’s Law of Octaves, Dobereiner’s Triads • Mendeleev’s Periodic table (based on atomic mass), holes in his table • Moseley, atomic number, protons, Modern Periodic Law • basic characteristics and names of the major groups • metals, nonmetals, metalloids – “stairca ...
AS Paper 1 Practice Paper 4 - A
... The student decided to use a measuring cylinder to obtain 25.0 cm3 of the supplier’s solution. This was poured into a 250 cm3 graduated flask and the liquid level was made up to the mark with de-ionised water. The student filled a burette with the acid solution. The following results were obtained w ...
... The student decided to use a measuring cylinder to obtain 25.0 cm3 of the supplier’s solution. This was poured into a 250 cm3 graduated flask and the liquid level was made up to the mark with de-ionised water. The student filled a burette with the acid solution. The following results were obtained w ...
GENERAL CHEMISTRY SECTION IV: THERMODYNAMICS
... 1. Raising the temperature increases ΔS. Why? – When heat increases, kinetic energy goes up, making velocity go up, and this molecules end up separating more frequently. 2. Increasing the volume increases ΔS. Why? – Molecules bouncing around in a cup will become more disordered if they’re moved and ...
... 1. Raising the temperature increases ΔS. Why? – When heat increases, kinetic energy goes up, making velocity go up, and this molecules end up separating more frequently. 2. Increasing the volume increases ΔS. Why? – Molecules bouncing around in a cup will become more disordered if they’re moved and ...
3.091 Summary Lecture Notes, Fall 2009
... o It’s a defining equation for quantum mechanics o Think of it as equivalent to Newton’s equation: F=ma o Complex equation that allows us to calculate measurable quantities, such as position, momentum, energy of microscopic systems. o Well beyond the scope of this class… ...
... o It’s a defining equation for quantum mechanics o Think of it as equivalent to Newton’s equation: F=ma o Complex equation that allows us to calculate measurable quantities, such as position, momentum, energy of microscopic systems. o Well beyond the scope of this class… ...
Fall 2012 Chem106 Final Review Name: Test 1 Materials Question
... Question 8. Absorbing a neutron will have what affect on the nucleus? a) increase atomic mass # by 2 a) increase atomic mass # by 1 a) decrease atomic mass # by 2 a) decrease atomic mass # by 1 Question 9. If the density of a liquid is 1.07g/mL, what volume does 32.4 g of it have? a) 30.28 mL b) 30. ...
... Question 8. Absorbing a neutron will have what affect on the nucleus? a) increase atomic mass # by 2 a) increase atomic mass # by 1 a) decrease atomic mass # by 2 a) decrease atomic mass # by 1 Question 9. If the density of a liquid is 1.07g/mL, what volume does 32.4 g of it have? a) 30.28 mL b) 30. ...
Homework,1 Atoms, molecules, and ions
... and by how much (assuming the reaction goes to completion)? a) A is in excess by 0.333 mol. b) B is in excess by 0.333 mol. c) B is in excess by 0.250 mol. d) Neither A nor B is in excess, because the reaction "goes to completion." e) A is in excess by 0.250 mol. 8- For the reaction Fe(CO)5 + 2PF3 + ...
... and by how much (assuming the reaction goes to completion)? a) A is in excess by 0.333 mol. b) B is in excess by 0.333 mol. c) B is in excess by 0.250 mol. d) Neither A nor B is in excess, because the reaction "goes to completion." e) A is in excess by 0.250 mol. 8- For the reaction Fe(CO)5 + 2PF3 + ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Chapter 2: Atoms, Ions, and the Periodic Table
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
... A) Pure water is composed of the elements oxygen and hydrogen in a mass ratio of 8 to 1. B) Any sample of a given compound always contains the same proportions by mass of the component elements. C) The mass of the products of a chemical reaction is equal to the mass of the starting materials of the ...
Atomic Physics Sections 9.1-9.7
... Bohr and the Hydrogen Atom • Bohr predicted that the single hydrogen electron would only be found in discrete orbits with particular radii – Bohr’s possible electron orbits were given wholenumber designations, n = 1, 2, 3, … – “n” is called the principal quantum number – The lowest n-value, (n = 1) ...
... Bohr and the Hydrogen Atom • Bohr predicted that the single hydrogen electron would only be found in discrete orbits with particular radii – Bohr’s possible electron orbits were given wholenumber designations, n = 1, 2, 3, … – “n” is called the principal quantum number – The lowest n-value, (n = 1) ...
Summer Work
... Third Exercise: Writing the balanced ionic Equation, predict the products for the following solutions are combined. Circle the precipitate (if any), place a box around the spectator ions. a. potassium chloride(aq) + silver(I) nitrate(aq) → b. lead (II) nitrate(aq) + hydrogen chloride(aq) → c. sodium ...
... Third Exercise: Writing the balanced ionic Equation, predict the products for the following solutions are combined. Circle the precipitate (if any), place a box around the spectator ions. a. potassium chloride(aq) + silver(I) nitrate(aq) → b. lead (II) nitrate(aq) + hydrogen chloride(aq) → c. sodium ...
3 ATOMIC STRUCTURE C MODULE - 2
... approximately 1840 times heavier than an electron. Further’ experiments revealed that the atomic masses were more than that expected from the presence of just protons and electrons in the atom. For example, the mass of helium atom was expected to be double that of hydrogen atom but was actually foun ...
... approximately 1840 times heavier than an electron. Further’ experiments revealed that the atomic masses were more than that expected from the presence of just protons and electrons in the atom. For example, the mass of helium atom was expected to be double that of hydrogen atom but was actually foun ...
The Mole
... Chemists often need to know about the quantitative relationships among the elements and compounds involved in a chemical reaction. These relationships may involve masses and or volumes. For Example, a chemist might want to know what volume of gas is produced when a certain mass of a compound is heat ...
... Chemists often need to know about the quantitative relationships among the elements and compounds involved in a chemical reaction. These relationships may involve masses and or volumes. For Example, a chemist might want to know what volume of gas is produced when a certain mass of a compound is heat ...
AP Chemistry Standards and Benchmarks
... These descriptive facts, including chemistry involved in environmental and societal issues, should not be isolated form the principles being studied but should be taught throughout the course to illustrate and illuminate the principles. The following areas should be covered: • chemical reactivity an ...
... These descriptive facts, including chemistry involved in environmental and societal issues, should not be isolated form the principles being studied but should be taught throughout the course to illustrate and illuminate the principles. The following areas should be covered: • chemical reactivity an ...
Development of the Atomic Theory
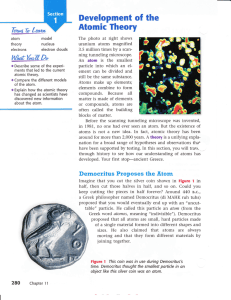
... the nucleus, as shown in the model in Figure tI. This is an atom of the element helium. Building Bigger Atoms You could build a carbon atom using 6 protons, 6 neutrons, and 6 electrons; or you could build an oxygen atom using 8 protons, 9 neutrons, and 8 electrons. You could even build a gold atom w ...
... the nucleus, as shown in the model in Figure tI. This is an atom of the element helium. Building Bigger Atoms You could build a carbon atom using 6 protons, 6 neutrons, and 6 electrons; or you could build an oxygen atom using 8 protons, 9 neutrons, and 8 electrons. You could even build a gold atom w ...
1411 Practice Exam 1
... Carry out the following operations and give your answers with the correct number of significant figures (2 pts each): a) 14 + 6.724 + 0.0099 ...
... Carry out the following operations and give your answers with the correct number of significant figures (2 pts each): a) 14 + 6.724 + 0.0099 ...
Chemistry 1411 Practice Exam 1, Chapters 1
... When an object claimed to be made of pure gold was immersed in a graduated cylinder containing water, the water level rose from 25.0 mL to 25.8 mL. The mass of the object was 7.1 g. What is the density of the object in grams per mL? (3 pts) ...
... When an object claimed to be made of pure gold was immersed in a graduated cylinder containing water, the water level rose from 25.0 mL to 25.8 mL. The mass of the object was 7.1 g. What is the density of the object in grams per mL? (3 pts) ...
File
... o 3.A.2 Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done by only chemists. ...
... o 3.A.2 Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done by only chemists. ...
Chapter 7 – Chemical Reactions and Energy Flow
... individual molecules of water accelerate and move much rapidly within the solution. Some have sufficient kinetic energy that they are capable of overcoming their intermolecular attractions and leave the solution. 2) A change in potential energy. The molecules, on average, move further apart (which i ...
... individual molecules of water accelerate and move much rapidly within the solution. Some have sufficient kinetic energy that they are capable of overcoming their intermolecular attractions and leave the solution. 2) A change in potential energy. The molecules, on average, move further apart (which i ...
C6-Chemical Reactions
... Group- Vertical columns on the periodic table Elements within a group have similar chemical and physical ...
... Group- Vertical columns on the periodic table Elements within a group have similar chemical and physical ...
Monday, Sept. 24, 2012
... Pieces of evidence that scientists had in 1900 to indicate that the atom was not a fundamental unit There are simply too many kinds of atoms (~70 known at that time), belonging to a distinct chemical element ...
... Pieces of evidence that scientists had in 1900 to indicate that the atom was not a fundamental unit There are simply too many kinds of atoms (~70 known at that time), belonging to a distinct chemical element ...
Classification of Matter
... Properties of Matter Physical vs. Chemical Properties • Physical properties can be measure without changing the basic identity of the substance (e.g., color, density, odor, melting point) • Chemical properties describe how substances react or change to form different substances (e.g., hydrogen bur ...
... Properties of Matter Physical vs. Chemical Properties • Physical properties can be measure without changing the basic identity of the substance (e.g., color, density, odor, melting point) • Chemical properties describe how substances react or change to form different substances (e.g., hydrogen bur ...