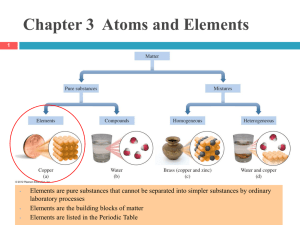
Classification of Matter
... Properties of Matter Physical vs. Chemical Properties • Physical properties can be measure without changing the basic identity of the substance (e.g., color, density, odor, melting point) • Chemical properties describe how substances react or change to form different substances (e.g., hydrogen bur ...
... Properties of Matter Physical vs. Chemical Properties • Physical properties can be measure without changing the basic identity of the substance (e.g., color, density, odor, melting point) • Chemical properties describe how substances react or change to form different substances (e.g., hydrogen bur ...
Common Curriculum Map Discipline: Science Course: Chemistry
... 2. List the 4 postulates of the atomic theory of matter. 3. List the three main parts of an atom, along with each part’s electrical charge. 4. Discuss the relative size and mass of protons, electrons, and neutrons. 5. Use the periodic table to determine the atomic number of a given element. 6. Expla ...
... 2. List the 4 postulates of the atomic theory of matter. 3. List the three main parts of an atom, along with each part’s electrical charge. 4. Discuss the relative size and mass of protons, electrons, and neutrons. 5. Use the periodic table to determine the atomic number of a given element. 6. Expla ...
Chemistry - Summative Practice and Review for Chapter 4 and 5
... ____ 17. An element has an atomic number of 76. The number of protons and electrons in a neutral atom of the element are ____. a. 152 protons and 76 electrons c. 38 protons and 38 electrons b. 76 protons and 0 electrons d. 76 protons and 76 electrons ____ 18. The mass number of an element is equal t ...
... ____ 17. An element has an atomic number of 76. The number of protons and electrons in a neutral atom of the element are ____. a. 152 protons and 76 electrons c. 38 protons and 38 electrons b. 76 protons and 0 electrons d. 76 protons and 76 electrons ____ 18. The mass number of an element is equal t ...
xmas review questions 01516 with hints
... 17. Predicts that it is impossible to determine simultaneously the exact position and the exact velocity of an electron Because an electron has both wave and particle properties, the more we know about one quality the less we know about the other. ...
... 17. Predicts that it is impossible to determine simultaneously the exact position and the exact velocity of an electron Because an electron has both wave and particle properties, the more we know about one quality the less we know about the other. ...
- TestbankU
... 5) Why is it so difficult to remove the lid from a vacuum-sealed jar? A) The air pressure outside the jar pushes downward on the lid more strongly than the air pressure inside pushes upward on the lid. B) The vacuum inside the jar pushes outward on the lid, holding it firmly to the jar. C) The vacuu ...
... 5) Why is it so difficult to remove the lid from a vacuum-sealed jar? A) The air pressure outside the jar pushes downward on the lid more strongly than the air pressure inside pushes upward on the lid. B) The vacuum inside the jar pushes outward on the lid, holding it firmly to the jar. C) The vacuu ...
Test 9 Review - Evan`s Chemistry Corner
... Collision theory. In order for a reaction to occur, particles of the reactant must collide. Not all collisions cause reactions. An effective collision is one in which the colliding particles approach each other at the proper angle and with the proper amount of energy to cause a reaction. The greater ...
... Collision theory. In order for a reaction to occur, particles of the reactant must collide. Not all collisions cause reactions. An effective collision is one in which the colliding particles approach each other at the proper angle and with the proper amount of energy to cause a reaction. The greater ...
Word Document
... 2. Which molecule is the least stable (has the highest energy) cis-1,3-dimethylcyclohexane or trans-1,3dimethyl cyclohexane. Explain 3. What is the configuration of the product of the reaction of bromine with cis-2-pentene? 1. Given the proton NMR spectrum, 13C NMR spectrum, mass spectroscopy peaks, ...
... 2. Which molecule is the least stable (has the highest energy) cis-1,3-dimethylcyclohexane or trans-1,3dimethyl cyclohexane. Explain 3. What is the configuration of the product of the reaction of bromine with cis-2-pentene? 1. Given the proton NMR spectrum, 13C NMR spectrum, mass spectroscopy peaks, ...
AP `99 Multiple Choice
... (B) Evaporation to dryness 50. In the periodic table, as the atomic number increases from 11 to 17, what happens to the atomic radius? (A) It remains constant. (B) It increases only. (C) It increases, then decreases. (D) It decreases only. (E) It decreases, then increases. 51. Which of the following ...
... (B) Evaporation to dryness 50. In the periodic table, as the atomic number increases from 11 to 17, what happens to the atomic radius? (A) It remains constant. (B) It increases only. (C) It increases, then decreases. (D) It decreases only. (E) It decreases, then increases. 51. Which of the following ...
Describing Chemical Reactions
... of a reaction are present at the end. ________________________ 10. At the end of a chemical reaction, what is the total mass of the reactants compared to the total mass of the products? ...
... of a reaction are present at the end. ________________________ 10. At the end of a chemical reaction, what is the total mass of the reactants compared to the total mass of the products? ...
1999 Advanced Placement Chemistry Exam
... (B) Evaporation to dryness 50. In the periodic table, as the atomic number increases from 11 to 17, what happens to the atomic radius? (A) It remains constant. (B) It increases only. (C) It increases, then decreases. (D) It decreases only. (E) It decreases, then increases. 51. Which of the following ...
... (B) Evaporation to dryness 50. In the periodic table, as the atomic number increases from 11 to 17, what happens to the atomic radius? (A) It remains constant. (B) It increases only. (C) It increases, then decreases. (D) It decreases only. (E) It decreases, then increases. 51. Which of the following ...
Chapter 3 Notes PowerPoint
... If the magma cools very slowly it forms large crystals. If it cools very quickly it forms tiny to microscopic crystals. ...
... If the magma cools very slowly it forms large crystals. If it cools very quickly it forms tiny to microscopic crystals. ...
avogadro exam 1994 - University of Waterloo
... 9. Which neutral halogen atom has the smallest ionization energy for the first electron removed? ...
... 9. Which neutral halogen atom has the smallest ionization energy for the first electron removed? ...
Types of Chemical Reactions
... table. What do these represent? E.g. the atomic mass of C is 12 (atomic # is 6) We know there are 6 protons and 6 neutrons Protons and neutrons have roughly the same mass. So, C weighs 12 u (atomic mass units). What is the actual mass of a C atom? Answer: approx. 2 x 10-23 grams (protons a ...
... table. What do these represent? E.g. the atomic mass of C is 12 (atomic # is 6) We know there are 6 protons and 6 neutrons Protons and neutrons have roughly the same mass. So, C weighs 12 u (atomic mass units). What is the actual mass of a C atom? Answer: approx. 2 x 10-23 grams (protons a ...
KS4-Chemical-Reactions
... 2. Increasing the pressure in gas reactions favours whichever side of the chemical equation has least gas ...
... 2. Increasing the pressure in gas reactions favours whichever side of the chemical equation has least gas ...
Chapter 8
... 5. These charts help to keep track of which elements are balanced in a chemical equation. The charts are one way of keeping track of the number of atoms of each element on the reactant side of a chemical equation and on the product side of an equation. The top row in a chart gives the number and typ ...
... 5. These charts help to keep track of which elements are balanced in a chemical equation. The charts are one way of keeping track of the number of atoms of each element on the reactant side of a chemical equation and on the product side of an equation. The top row in a chart gives the number and typ ...
Data: I am writing out the question and underlining it.
... thought of where all the statistics we hear about come from, and how the claims are substantiated. How do scientists know exactly what percent our ozone layer has deteriorated, and what percent of our atmosphere is made up harmful pollutants? Well when fossil fuels are burned, or maybe even things l ...
... thought of where all the statistics we hear about come from, and how the claims are substantiated. How do scientists know exactly what percent our ozone layer has deteriorated, and what percent of our atmosphere is made up harmful pollutants? Well when fossil fuels are burned, or maybe even things l ...
3.Masses of individual atoms
... composition(number of protons ,electrons, neutrons),but also in mass, Chemical formulas of compounds tell us not only the atom ratios in which elements are present but also the mass ratios. ...
... composition(number of protons ,electrons, neutrons),but also in mass, Chemical formulas of compounds tell us not only the atom ratios in which elements are present but also the mass ratios. ...
Chemistry - Sanskriti School
... 2) Classification of substances into elements, compounds and mixtures. Experiment: To mix iron and sulphur physically and study its properties and then by heating the mixture study the change in properties. 3) Difference between compounds and mixtures. 4) Heterogeneous and homogeneous mixtures. Acti ...
... 2) Classification of substances into elements, compounds and mixtures. Experiment: To mix iron and sulphur physically and study its properties and then by heating the mixture study the change in properties. 3) Difference between compounds and mixtures. 4) Heterogeneous and homogeneous mixtures. Acti ...
Second exam 2014 with answers
... Fill in your answer in the blank space provided immediately following each question. 1/2 point will be subtracted every time you report a numerical result with an incorrect number of significant figures. The last sheet of this exam contains data that may be needed to answer these questions. R= 0.082 ...
... Fill in your answer in the blank space provided immediately following each question. 1/2 point will be subtracted every time you report a numerical result with an incorrect number of significant figures. The last sheet of this exam contains data that may be needed to answer these questions. R= 0.082 ...
+ H 2 (g) - WordPress.com
... Standard Enthalpies of Formation The term standard state refers to the standard thermodynamic conditions chosen for substances when listing or comparing thermodynamic data: 1 atm pressure and the specified temperature (usually 25°C). These standard conditions are indicated with a degree sign (°). W ...
... Standard Enthalpies of Formation The term standard state refers to the standard thermodynamic conditions chosen for substances when listing or comparing thermodynamic data: 1 atm pressure and the specified temperature (usually 25°C). These standard conditions are indicated with a degree sign (°). W ...
Notes: Kinetics and Equilibrium
... Spontaneous reactions will happen with no outside influence. An everyday example would be a battery. You simple allow the ends of a battery to touch and a chemical reaction will occur. The reaction is called an electrochemical reaction, as electrons move from one substance to another. These substanc ...
... Spontaneous reactions will happen with no outside influence. An everyday example would be a battery. You simple allow the ends of a battery to touch and a chemical reaction will occur. The reaction is called an electrochemical reaction, as electrons move from one substance to another. These substanc ...
CHAPTER 8: ENERGY FROM ELECTRON TRANSFER
... the semiconductor) is surrounded by 9 electrons. Silicon is in Group 4A and has 4 outer electrons. Thus central atom in the figure must have 5 outer electrons. This is consistent with an element in Group 5A such as arsenic, so this is an n-type silicon semiconductor. 28. Describe the main reasons wh ...
... the semiconductor) is surrounded by 9 electrons. Silicon is in Group 4A and has 4 outer electrons. Thus central atom in the figure must have 5 outer electrons. This is consistent with an element in Group 5A such as arsenic, so this is an n-type silicon semiconductor. 28. Describe the main reasons wh ...