What is the difference between atomic mass and atomic number?
... •F0J5e. 10. Atomic number atomic mass. ...
... •F0J5e. 10. Atomic number atomic mass. ...
Chemistry (306) - National Evaluation Series
... bedding, automobile seats, and sponges. An important component in the manufacturing of flexible polyurethane foams are polyols, which are typically derived from petroleum products. In 2007, Cargill, Incorporated, was awarded a Presidential Green Chemistry Challenge Award from the Environmental Prote ...
... bedding, automobile seats, and sponges. An important component in the manufacturing of flexible polyurethane foams are polyols, which are typically derived from petroleum products. In 2007, Cargill, Incorporated, was awarded a Presidential Green Chemistry Challenge Award from the Environmental Prote ...
- Kendriya Vidyalaya No. 2 Raipur
... Mohan took pure water for the electrolytic decomposition of water but did not see any bubbles near the electrodes. Explain why? Rancidity is a process used for spoiling of cooked food materials like vegetables, etc. When kept for long time in open. How can you prevent such process to proceed? Give a ...
... Mohan took pure water for the electrolytic decomposition of water but did not see any bubbles near the electrodes. Explain why? Rancidity is a process used for spoiling of cooked food materials like vegetables, etc. When kept for long time in open. How can you prevent such process to proceed? Give a ...
File
... Mohan took pure water for the electrolytic decomposition of water but did not see any bubbles near the electrodes. Explain why? Rancidity is a process used for spoiling of cooked food materials like vegetables, etc. When kept for long time in open. How can you prevent such process to proceed? Give a ...
... Mohan took pure water for the electrolytic decomposition of water but did not see any bubbles near the electrodes. Explain why? Rancidity is a process used for spoiling of cooked food materials like vegetables, etc. When kept for long time in open. How can you prevent such process to proceed? Give a ...
File - Meissnerscience.com
... a water solution and has _____ molecules incorporated into their crystal structure. Hydrates have a ________________ of water molecules ___________ bonded to each _________________. Compounds that have no water molecules incorporated into them are called _________________ to distinguish them from th ...
... a water solution and has _____ molecules incorporated into their crystal structure. Hydrates have a ________________ of water molecules ___________ bonded to each _________________. Compounds that have no water molecules incorporated into them are called _________________ to distinguish them from th ...
CHAPTER 20 METALLURGY AND THE CHEMISTRY OF METALS
... The reaction in part (a) shows us that three moles of electrons are required to produce one mole of aluminum. The voltage is three times the minimum calculated above (namely, −3.09 V or −3.09 J/C). We can find the electrical energy by using the same equation with the other voltage. ...
... The reaction in part (a) shows us that three moles of electrons are required to produce one mole of aluminum. The voltage is three times the minimum calculated above (namely, −3.09 V or −3.09 J/C). We can find the electrical energy by using the same equation with the other voltage. ...
1-4 What Are The Parts Of An Atom and How Are They Arranged
... Thomson thought about his results for a long time. It was almost as if the cathode rays were attracted to the positively charged metal plate and repelled from the negatively charged metal plate. Thomson knew that charged objects are attracted to and repelled from other charged objects according to ...
... Thomson thought about his results for a long time. It was almost as if the cathode rays were attracted to the positively charged metal plate and repelled from the negatively charged metal plate. Thomson knew that charged objects are attracted to and repelled from other charged objects according to ...
Chapter 3 Molecules, Compounds, and Chemical Equations
... Symbols Used in Equations - several symbols are used in chemical equations i.) symbols used to indicate state after chemical (g) = gas; (l) = liquid; (s) = solid (aq) = aqueous = dissolved in water gy symbols y used above the arrow for decomposition p ii.)) energy reactions = heat h = lig ...
... Symbols Used in Equations - several symbols are used in chemical equations i.) symbols used to indicate state after chemical (g) = gas; (l) = liquid; (s) = solid (aq) = aqueous = dissolved in water gy symbols y used above the arrow for decomposition p ii.)) energy reactions = heat h = lig ...
Relative atomic and molecular mass
... Atomic Mass Units The actual mass of a hydrogen atom is 1.7x10-24g (that’s 0.0000000000000000000000017g!) Far too small a number to easily get your head around… So – we use Relative Atomic Masses. ...
... Atomic Mass Units The actual mass of a hydrogen atom is 1.7x10-24g (that’s 0.0000000000000000000000017g!) Far too small a number to easily get your head around… So – we use Relative Atomic Masses. ...
Chapter 3 Molecules, Compounds, and Chemical Equations q
... - a compound is a distinct substance that is composed of atoms of two or more elements. - describe the compound by describing the number and type of each atom in the simplest unit of the compound. molecules or ions - each element is represented by its letter symbol. - the number of atoms of each e ...
... - a compound is a distinct substance that is composed of atoms of two or more elements. - describe the compound by describing the number and type of each atom in the simplest unit of the compound. molecules or ions - each element is represented by its letter symbol. - the number of atoms of each e ...
File
... SCH4U b) Determine the oxidation number of the sulfur in the sulfate ion, SO4-2. Remember that the sum of the oxidation numbers must equal the charge of the ion. Step 1: Write All Known Oxidation Numbers Use the symbol N to represent the oxidation number of sulfur in the sulfate. ...
... SCH4U b) Determine the oxidation number of the sulfur in the sulfate ion, SO4-2. Remember that the sum of the oxidation numbers must equal the charge of the ion. Step 1: Write All Known Oxidation Numbers Use the symbol N to represent the oxidation number of sulfur in the sulfate. ...
Chapter 2 Atoms, Molecules, and Ions
... Ø Elements are represented by a one or two letter symbol. This is the symbol for carbon. Ø All atoms of the same element have the same number of protons, which is called the atomic number, Z. It is written as a subscript BEFORE the symbol. Ø The mass number is the total number of protons and n ...
... Ø Elements are represented by a one or two letter symbol. This is the symbol for carbon. Ø All atoms of the same element have the same number of protons, which is called the atomic number, Z. It is written as a subscript BEFORE the symbol. Ø The mass number is the total number of protons and n ...
physical setting chemistry
... (3) negative ion with a radius smaller than the radius of this atom (4) negative ion with a radius larger than the radius of this atom ...
... (3) negative ion with a radius smaller than the radius of this atom (4) negative ion with a radius larger than the radius of this atom ...
2008 local exam - American Chemical Society
... increases. This is because the (A) activation energy for the process is lowered. (B) average kinetic energy of the reactants increases. (C) higher temperature catalyzes the reaction. (D) higher temperature changes the wavelength of light ...
... increases. This is because the (A) activation energy for the process is lowered. (B) average kinetic energy of the reactants increases. (C) higher temperature catalyzes the reaction. (D) higher temperature changes the wavelength of light ...
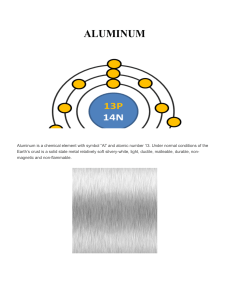
ALUMINUM
... The nucleosynthesis of Aluminum arises in heavy stars when a hydrogen proton is added to magnesium nuclei (which in turn consists of two carbon nuclear fusion) in large stars and supernovae. ...
... The nucleosynthesis of Aluminum arises in heavy stars when a hydrogen proton is added to magnesium nuclei (which in turn consists of two carbon nuclear fusion) in large stars and supernovae. ...
CBSE-12th/2011/CHEMISTRY
... (ii)O=O is a much stronger bond than O-O (about 3 times). Also, O has a small size. S is larger in size. so lp repulsion is less significant. Also, S-S bond is stronger than O-O bond & S=S is less strong(less than 2 S-S bonds). This is also affected by the fact that O forms strong bonds with mostly ...
... (ii)O=O is a much stronger bond than O-O (about 3 times). Also, O has a small size. S is larger in size. so lp repulsion is less significant. Also, S-S bond is stronger than O-O bond & S=S is less strong(less than 2 S-S bonds). This is also affected by the fact that O forms strong bonds with mostly ...
Atomic Theory Timeline - My Extra Help Teacher
... The scientists included in this timeline are: Democritus/Aristotle, Alchemists, Dalton, Becquerel, Thomson, Millikan, Rutherford, Bohr, Schrödinger, and Chadwick. As the teacher, you may choose to omit some of these or cover them yourself. Democritus/Aristotle and Alchemists are easily covered as an ...
... The scientists included in this timeline are: Democritus/Aristotle, Alchemists, Dalton, Becquerel, Thomson, Millikan, Rutherford, Bohr, Schrödinger, and Chadwick. As the teacher, you may choose to omit some of these or cover them yourself. Democritus/Aristotle and Alchemists are easily covered as an ...
Ch. 3 Sections 3.9-3.10 Notes
... • Some reactions proceed better when one reactant is in stoichiometric excess, for example. • One such reaction is the preparation of ammonia, NH3, from its elements. ...
... • Some reactions proceed better when one reactant is in stoichiometric excess, for example. • One such reaction is the preparation of ammonia, NH3, from its elements. ...
Inside the atom - Oxford University Press
... near the English Lake District. Because Dalton was part of the Quaker tradition (Quakers did not follow the teachings of the ‘establishment’ Church of England), he was not able to attend or teach at an English university. Instead, when he was 27, he taught mathematics and natural philosophy at a col ...
... near the English Lake District. Because Dalton was part of the Quaker tradition (Quakers did not follow the teachings of the ‘establishment’ Church of England), he was not able to attend or teach at an English university. Instead, when he was 27, he taught mathematics and natural philosophy at a col ...
Introductory Chemistry The Evolution of Atomic Theory
... • Protons—relatively massive and positively (+1) ...
... • Protons—relatively massive and positively (+1) ...
CHM 22 Test 2Take-homeKey Student Name
... 9. The following reaction: Mg + FeO MgO + Fe, is an example of A. combination. B. cecomposition. C. single-displacement. D. double-displacement. Answer: C; Difficulty: easy; Reference: Section 8.4 10. The following reaction: NaOH + HCl NaCl + H2O, is an example of A. combination. B. decomposition. C ...
... 9. The following reaction: Mg + FeO MgO + Fe, is an example of A. combination. B. cecomposition. C. single-displacement. D. double-displacement. Answer: C; Difficulty: easy; Reference: Section 8.4 10. The following reaction: NaOH + HCl NaCl + H2O, is an example of A. combination. B. decomposition. C ...
Dalton`s Atomic Theory
... many of these atoms must be lined up in a row to produce a line 1 m long? •1 1010 (10,000,000,000) atoms of radius 1 10-10 m would need to be lined up in a row to produce a line 1 m long. Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... many of these atoms must be lined up in a row to produce a line 1 m long? •1 1010 (10,000,000,000) atoms of radius 1 10-10 m would need to be lined up in a row to produce a line 1 m long. Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
Time
... The Ksp for scandium fluoride (ScF3) at 298 K is 4.2 x 10-18 Write the chemical equation for the solubility equilibrium of scandium fluoride in water. What concentration of Sc3+ ions is required to cause a precipitate to form if the fluoride ion concentration in a solution is 0.076 M ? ...
... The Ksp for scandium fluoride (ScF3) at 298 K is 4.2 x 10-18 Write the chemical equation for the solubility equilibrium of scandium fluoride in water. What concentration of Sc3+ ions is required to cause a precipitate to form if the fluoride ion concentration in a solution is 0.076 M ? ...