PeriodicTableA
... as well as s electrons, allowing for multiple oxidation states. Most d Block elements have a +2 oxidation State which corresponds to the loss of the two s electrons. This is especially true on the right side of the d block, but less true on the left. ---- For example Sc+2 does not exist, and Ti+2 is ...
... as well as s electrons, allowing for multiple oxidation states. Most d Block elements have a +2 oxidation State which corresponds to the loss of the two s electrons. This is especially true on the right side of the d block, but less true on the left. ---- For example Sc+2 does not exist, and Ti+2 is ...
Chemistry Periodic Table and Trends Periodic Table The periodic
... Atomic Radius: Atomic radius is measured from the center of the atom to the outermost orbital shell of the atom. It increases going down groups of the periodic table because energy shells are added to accommodate more electrons. For instance, Hydrogen (H), Lithium (Li) and Sodium (Na) are in the sam ...
... Atomic Radius: Atomic radius is measured from the center of the atom to the outermost orbital shell of the atom. It increases going down groups of the periodic table because energy shells are added to accommodate more electrons. For instance, Hydrogen (H), Lithium (Li) and Sodium (Na) are in the sam ...
Organizing the periodic table
... increases. The lightest atom is Hydrogen, the heaviest is Ununoctium, atomic number 118. ...
... increases. The lightest atom is Hydrogen, the heaviest is Ununoctium, atomic number 118. ...
Chapter 5 Section 3: Electron Configuration and Periodic Properties
... energy. When energy is absorbed it is represented by a positive number and the ion is unstable and usually decays (breaks apart back to the element). Periodic Trend for Electron Affinity – It is easier to add ...
... energy. When energy is absorbed it is represented by a positive number and the ion is unstable and usually decays (breaks apart back to the element). Periodic Trend for Electron Affinity – It is easier to add ...
UNIT 4 NOTES: THE PERIODIC TABLE
... 2. They are __________ at reactive as other metals. 3. They form _______________ solutions when dissolved in water. For example Cu+2 is ___________, while Fe3+ is ___________. In other words transition elements have _______________ ions. 4. They have _____________________ positive oxidation states. ...
... 2. They are __________ at reactive as other metals. 3. They form _______________ solutions when dissolved in water. For example Cu+2 is ___________, while Fe3+ is ___________. In other words transition elements have _______________ ions. 4. They have _____________________ positive oxidation states. ...
The Periodic Table
... Boron Family • Elements in group 13 • Aluminum metal was once rare and expensive as Gold. Not a “disposable metal.” • Has now been discovered in a very ...
... Boron Family • Elements in group 13 • Aluminum metal was once rare and expensive as Gold. Not a “disposable metal.” • Has now been discovered in a very ...
January Exam Review
... 1. In general, in the periodic table, the atomic masses of elements increase as their atomic numbers increase. There are, however, exceptions to this rule. Which of the following accounts for these exceptions? A) The number of electrons increases irregularly. B) The number of particles in the nucleu ...
... 1. In general, in the periodic table, the atomic masses of elements increase as their atomic numbers increase. There are, however, exceptions to this rule. Which of the following accounts for these exceptions? A) The number of electrons increases irregularly. B) The number of particles in the nucleu ...
Chemistry Online Textbook
... total. Only 255 of these naturally occurring isotopes are stable in the sense of never having been observed to decay as of the present time. All the known stable isotopes occur naturally on Earth; the other naturally occurring-isotopes are radioactive but occur on Earth due to their relatively long ...
... total. Only 255 of these naturally occurring isotopes are stable in the sense of never having been observed to decay as of the present time. All the known stable isotopes occur naturally on Earth; the other naturally occurring-isotopes are radioactive but occur on Earth due to their relatively long ...
Chapter 3 Atoms and Elements
... A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32S ...
... A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32S ...
Electrons in Atoms - Effingham County Schools
... Two electrons are in the s sublevel, so two electrons must also be present in the 2p sublevel, The outer electron configuration is 2s22p2 ...
... Two electrons are in the s sublevel, so two electrons must also be present in the 2p sublevel, The outer electron configuration is 2s22p2 ...
Period
... 11. Elements of Group 1 are called ________________________________________ 12. Elements of Group 2 are called ________________________________________ 13. Elements of Group 3-12 are called _____________________________________ 14. As you go from left to right across the periodic table, the elements ...
... 11. Elements of Group 1 are called ________________________________________ 12. Elements of Group 2 are called ________________________________________ 13. Elements of Group 3-12 are called _____________________________________ 14. As you go from left to right across the periodic table, the elements ...
Spring Final Exam Study Guide
... 31. An underground cavern contains 98 moles of methane gas at a pressure of 15atm and a temperature of 22C. How many liters of methane does this natural gas deposit contain? ...
... 31. An underground cavern contains 98 moles of methane gas at a pressure of 15atm and a temperature of 22C. How many liters of methane does this natural gas deposit contain? ...
60. Write the electron configuration for Zn
... 31. An underground cavern contains 98 moles of methane gas at a pressure of 15atm and a temperature of 22C. How many liters of methane does this natural gas deposit contain? ...
... 31. An underground cavern contains 98 moles of methane gas at a pressure of 15atm and a temperature of 22C. How many liters of methane does this natural gas deposit contain? ...
File
... 2. In each of the following pairs, pick the larger species. Explain you answer in each case. (a) Cu and Cu2+ (b) F and F(c) Na and K 3. Only one of the following statements is correct. Which one? (a) All cations are larger than their corresponding atoms (b) All anions are smaller than their correspo ...
... 2. In each of the following pairs, pick the larger species. Explain you answer in each case. (a) Cu and Cu2+ (b) F and F(c) Na and K 3. Only one of the following statements is correct. Which one? (a) All cations are larger than their corresponding atoms (b) All anions are smaller than their correspo ...
Groups and Families
... reactive metals, some Lanthanides andused to make steel Actinies: 2 rows Electrons in the Outer Shellat (Valence Electrons): 1 or 2 bottom of periodic Actinides: radioactive table Less to keep table (unstable) Elements after Reactivity: reactive than from being toooccur wide. plutonium do not in alk ...
... reactive metals, some Lanthanides andused to make steel Actinies: 2 rows Electrons in the Outer Shellat (Valence Electrons): 1 or 2 bottom of periodic Actinides: radioactive table Less to keep table (unstable) Elements after Reactivity: reactive than from being toooccur wide. plutonium do not in alk ...
Chapter 4 - ETSU.edu
... Mosley an England scientist worked with x-ray spectra to study atomic structure which helped him determine atomic numbers for chemical elements. He discovered isotopes, explaining how atomic mass did not order the elements appropriately. Moseley was the catalyst for the periodic law, a rule statin ...
... Mosley an England scientist worked with x-ray spectra to study atomic structure which helped him determine atomic numbers for chemical elements. He discovered isotopes, explaining how atomic mass did not order the elements appropriately. Moseley was the catalyst for the periodic law, a rule statin ...
to Ch 05 Periodic Trends
... the table: the position predicted by an element’s atomic mass did not always match the position predicted by its chemical properties. • Moseley redesigned the table based on the increasing number of p+ (atomic number). ...
... the table: the position predicted by an element’s atomic mass did not always match the position predicted by its chemical properties. • Moseley redesigned the table based on the increasing number of p+ (atomic number). ...
Unit 10 Lecture Notes
... a. Elements in the same group have similar chemical and physical properties b. Elements in different groups have different properties, even if they are in the same period 3. Density and boiling point, for example, are "periodic properties"; there are many others (next part of chapter!) Periodic Tren ...
... a. Elements in the same group have similar chemical and physical properties b. Elements in different groups have different properties, even if they are in the same period 3. Density and boiling point, for example, are "periodic properties"; there are many others (next part of chapter!) Periodic Tren ...
Ionic Compounds and Their Names
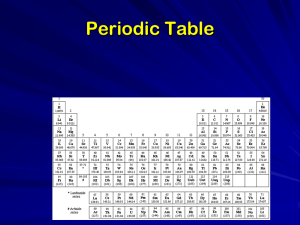
... E. Metalloids – have side along stair line (Excludes Al and most times, B) Group I A. Alkali Metals (Group 1) - form ions, each with a +1 charge II A. Alkaline Earth Metals (2) - tend to lose two electrons per atom, forming ions with a +2 charge The B Groups. Transition Metals (3-12) - consist of me ...
... E. Metalloids – have side along stair line (Excludes Al and most times, B) Group I A. Alkali Metals (Group 1) - form ions, each with a +1 charge II A. Alkaline Earth Metals (2) - tend to lose two electrons per atom, forming ions with a +2 charge The B Groups. Transition Metals (3-12) - consist of me ...
Learn About the Different Types of Elements on the Periodic Table
... Because they possess the properties of metals, the transition elements are also known as the transition metals. These elements are very hard, with high melting points and boiling points. Moving from left to right across the periodic table, the five d orbitals become more filled. The d electrons are ...
... Because they possess the properties of metals, the transition elements are also known as the transition metals. These elements are very hard, with high melting points and boiling points. Moving from left to right across the periodic table, the five d orbitals become more filled. The d electrons are ...
Better Understanding of Element Property Trends
... Property trend patterns - physical, electrical, or chemical aspects of adjacent elements of the periodic table - increase or decrease in different directions, but often not strictly continuous. The main trends are: ionization energy, electron affinity, metallic character, atomic radius, electronegat ...
... Property trend patterns - physical, electrical, or chemical aspects of adjacent elements of the periodic table - increase or decrease in different directions, but often not strictly continuous. The main trends are: ionization energy, electron affinity, metallic character, atomic radius, electronegat ...
Periodic Trend Notes
... Deduced elements existed, but were undiscovered elements, their properties could be predicted ...
... Deduced elements existed, but were undiscovered elements, their properties could be predicted ...
Worksheet 8-1 Periodic Trends
... Period 1. Discuss the importance of Mendeleev’s periodic law. When elements are arranged by increasing number of protons (atomic number), there are repeating patterns of chemical and physical properties. 2. Identify each element as a metal, metalloid, or nonmetal. a) Fluorine Nonmetal b) Germanium ...
... Period 1. Discuss the importance of Mendeleev’s periodic law. When elements are arranged by increasing number of protons (atomic number), there are repeating patterns of chemical and physical properties. 2. Identify each element as a metal, metalloid, or nonmetal. a) Fluorine Nonmetal b) Germanium ...
Question (1): Explain `Dobereiner`s Triads and its drawback. Answer
... Question (35): In the third period, which is the most metallic and non-metallic element. Answer: 1) The most metallic element is - Na (Sodium) 2) The most non-metallic is - Cl (Chlorine). Question (36): How does the number of valence shell change on moving 1) Down a group? 2) Down a period? Answer: ...
... Question (35): In the third period, which is the most metallic and non-metallic element. Answer: 1) The most metallic element is - Na (Sodium) 2) The most non-metallic is - Cl (Chlorine). Question (36): How does the number of valence shell change on moving 1) Down a group? 2) Down a period? Answer: ...