The Evolution of the Periodic System - Science
... to classify all the elements correctly. But the table did not appear in print until 1870 because of a publisher’s delay—a factor that contributed to an acrimonious dispute for priority that ensued between Lothar Meyer and Mendeleev. Around the same time, Mendeleev assembled his own periodic table wh ...
... to classify all the elements correctly. But the table did not appear in print until 1870 because of a publisher’s delay—a factor that contributed to an acrimonious dispute for priority that ensued between Lothar Meyer and Mendeleev. Around the same time, Mendeleev assembled his own periodic table wh ...
Particles and Periodic Table
... arrangement of electrons in its atoms and hence to its atomic number • predict possible reactions and probable reactivity of elements from their positions in the periodic table. Development of the periodic table Be able to describe the steps in the development of the Periodic Table: Before the disco ...
... arrangement of electrons in its atoms and hence to its atomic number • predict possible reactions and probable reactivity of elements from their positions in the periodic table. Development of the periodic table Be able to describe the steps in the development of the Periodic Table: Before the disco ...
Discovering Elements
... Once one electron shell is filled the next shell has to be started. For example, neon has 10 electrons, arranged 2,8. Since the second shell is now full, the next element sodium, with 11 electrons, begins a new shell. The electrons are arranged 2,8,1 and thus a new Period begins. As we move from left ...
... Once one electron shell is filled the next shell has to be started. For example, neon has 10 electrons, arranged 2,8. Since the second shell is now full, the next element sodium, with 11 electrons, begins a new shell. The electrons are arranged 2,8,1 and thus a new Period begins. As we move from left ...
PERIODIC TRENDS
... The Periodic Table was the outcome of several chemists working to make some sense out of the knowledge they were learning about the elements. John Newlands, Dmitri Mendeleev, and Henry Mosley all worked to give us the periodic table that we have today. John Newlands contribution to the periodic tabl ...
... The Periodic Table was the outcome of several chemists working to make some sense out of the knowledge they were learning about the elements. John Newlands, Dmitri Mendeleev, and Henry Mosley all worked to give us the periodic table that we have today. John Newlands contribution to the periodic tabl ...
The Periodic Table
... Less reactive than alkali metals, but still rarely found as free elements ...
... Less reactive than alkali metals, but still rarely found as free elements ...
Trends on the Periodic Table
... why the term periodic is used to describe the table. Example: periods of the moon ...
... why the term periodic is used to describe the table. Example: periods of the moon ...
H Unit 4: Periodic Table
... Activity: Color Coding the Periodic Table The Periodic Table is a list of all the known elements. It is organized by increasing atomic number. There are two main groups on the periodic table: metals and nonmetals. The left side of the table contains elements with the greatest metallic properties. As ...
... Activity: Color Coding the Periodic Table The Periodic Table is a list of all the known elements. It is organized by increasing atomic number. There are two main groups on the periodic table: metals and nonmetals. The left side of the table contains elements with the greatest metallic properties. As ...
Graphing Periodic Trends – Ana Julia Silva
... electronegativity. Electronegativity seems to increase across a period, going up from the alkali metals which all range at around one to the halogens with the highest values, until reaching the noble gases and crashing down to zero. 4a) What is happening to the number of protons and the number of en ...
... electronegativity. Electronegativity seems to increase across a period, going up from the alkali metals which all range at around one to the halogens with the highest values, until reaching the noble gases and crashing down to zero. 4a) What is happening to the number of protons and the number of en ...
ANSWERS-Review Trends in the Periodic Table
... Cations are always smaller than their neutral atoms. Many cations have lost an entire shell of electrons and are only about half the size of the neutral atom. Further decrease in ionic radius is due to the fact that cations have more protons in the nucleus than electrons. ...
... Cations are always smaller than their neutral atoms. Many cations have lost an entire shell of electrons and are only about half the size of the neutral atom. Further decrease in ionic radius is due to the fact that cations have more protons in the nucleus than electrons. ...
File
... Activity: Color Coding the Periodic Table The Periodic Table is a list of all the known elements. It is organized by increasing atomic number. There are two main groups on the periodic table: metals and nonmetals. The left side of the table contains elements with the greatest metallic properties. As ...
... Activity: Color Coding the Periodic Table The Periodic Table is a list of all the known elements. It is organized by increasing atomic number. There are two main groups on the periodic table: metals and nonmetals. The left side of the table contains elements with the greatest metallic properties. As ...
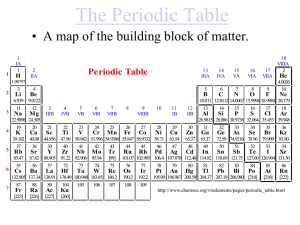
Elements are the building blocks of matter.
... 1. Which elements have outer shells that are full of electrons? Where are they located on the periodic table? 2. Which elements have only 1 electron in their outer shell? Where are they located on the periodic table? 3. What do you notice about the number of valence electrons (electrons in the outer ...
... 1. Which elements have outer shells that are full of electrons? Where are they located on the periodic table? 2. Which elements have only 1 electron in their outer shell? Where are they located on the periodic table? 3. What do you notice about the number of valence electrons (electrons in the outer ...
Modern Inorganic Chemistry
... On moving across a period, --- the atomic size decreases and hence the force of attraction exerted by the nucleus on the electrons increases. Consequently, the atom has a greater tendency to attract additional electron i.e., its electron affinity increases ...
... On moving across a period, --- the atomic size decreases and hence the force of attraction exerted by the nucleus on the electrons increases. Consequently, the atom has a greater tendency to attract additional electron i.e., its electron affinity increases ...
Periodic Table (Wiki)
... significant periodic trends than periods and blocks, explained below. Modern quantum mechanical theories of atomic structure explain group trends by proposing that elements within the same group generally have the same electron configurations in their valence shell.[8] Consequently, elements in the ...
... significant periodic trends than periods and blocks, explained below. Modern quantum mechanical theories of atomic structure explain group trends by proposing that elements within the same group generally have the same electron configurations in their valence shell.[8] Consequently, elements in the ...
Periodic Trends: Straw Lab
... To calculate the scale factor: Measure the length of a precut straw = ____________ cm Divide the length of the straw by 2 = ______________cm Divide that number by the largest value for ionization energy in the table This is your Scale factor=_______________ cm To calculate the length of your straw: ...
... To calculate the scale factor: Measure the length of a precut straw = ____________ cm Divide the length of the straw by 2 = ______________cm Divide that number by the largest value for ionization energy in the table This is your Scale factor=_______________ cm To calculate the length of your straw: ...
Chem1Unit4-7.14.15 - Grainger County Schools
... CLE 3221.1.2 Analyze the organization of the modern periodic table. 3221.1.4 Interpret a Bohr model of an electron moving between its ground and excited states in terms of the absorption or emission of energy. 3221.1.5 Use the periodic table to identify an element as a metal, nonmetal, or metalloid. ...
... CLE 3221.1.2 Analyze the organization of the modern periodic table. 3221.1.4 Interpret a Bohr model of an electron moving between its ground and excited states in terms of the absorption or emission of energy. 3221.1.5 Use the periodic table to identify an element as a metal, nonmetal, or metalloid. ...
Topic 3.2 Periodicity Physical Properties
... The Nuclear Charge (the number of protons) The effective nuclear charge is the relative number of protons in the nucleus to electrons it must interact with. The larger the ratio of protons to electrons, the more effective the nucleus will be and the relative result will be a smaller atomic radiu ...
... The Nuclear Charge (the number of protons) The effective nuclear charge is the relative number of protons in the nucleus to electrons it must interact with. The larger the ratio of protons to electrons, the more effective the nucleus will be and the relative result will be a smaller atomic radiu ...
Metals - Harding Charter Preparatory High School
... • Atoms of an element have different isotopes that occur at different frequencies • The atomic mass of an element is a weighted average mass of the atoms in a naturally occurring sample of the element – A weighted average mass reflects both the mass and the relative abundance of the isotopes as they ...
... • Atoms of an element have different isotopes that occur at different frequencies • The atomic mass of an element is a weighted average mass of the atoms in a naturally occurring sample of the element – A weighted average mass reflects both the mass and the relative abundance of the isotopes as they ...
The Periodic Table
... USING THE PERIODIC TABLE Rows are called periods. Columns are called groups. ...
... USING THE PERIODIC TABLE Rows are called periods. Columns are called groups. ...
Periodic Table - Mrs. Sousa`s Science Site
... Range of EN’s for the elements is 0.7 (Fr) to 4.0 (F) ...
... Range of EN’s for the elements is 0.7 (Fr) to 4.0 (F) ...
The Periodic Table
... and generally shiny; most are solid, ductile, and malleable. ductile: able to be drawn into wires malleable: able to be molded, ...
... and generally shiny; most are solid, ductile, and malleable. ductile: able to be drawn into wires malleable: able to be molded, ...
The Periodic Table: Chapter Problems Periodic Table Class Work
... b. Nitrogen has a smaller atomic radius than phosphorous because it has a lower principal quantum number than phosphorus. Phosphorous has a higher principal quantum number than so the electrons in the outer energy level are farther away from the nucleus. c. Nitrogen is more electronegative than phos ...
... b. Nitrogen has a smaller atomic radius than phosphorous because it has a lower principal quantum number than phosphorus. Phosphorous has a higher principal quantum number than so the electrons in the outer energy level are farther away from the nucleus. c. Nitrogen is more electronegative than phos ...
The Periodic Table
... Similarly, no two elements have the same atomic number. Atomic number refers to the number of protons in the nucleus of an atom. The sooty remains of burned logs are made up of a great deal of carbon. The element carbon has six protons and, therefore, has an atomic number of six. Nitrogen, a gas tha ...
... Similarly, no two elements have the same atomic number. Atomic number refers to the number of protons in the nucleus of an atom. The sooty remains of burned logs are made up of a great deal of carbon. The element carbon has six protons and, therefore, has an atomic number of six. Nitrogen, a gas tha ...
NAME - MrTestaScienceClass
... Graphing Periodic Trends Computer Activity – Academic Chemistry BACKGROUND: In 1870, Dmitri Mendeleev first proposed a new way of studying and organizing the then known 63 elements. The modern form of the table has been modified and improved many times since Mendeleev’s tables. Pioneers like Moseley ...
... Graphing Periodic Trends Computer Activity – Academic Chemistry BACKGROUND: In 1870, Dmitri Mendeleev first proposed a new way of studying and organizing the then known 63 elements. The modern form of the table has been modified and improved many times since Mendeleev’s tables. Pioneers like Moseley ...