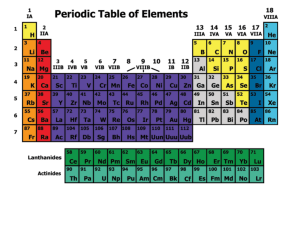
Chemical Matter: Elements and Their Classification
... changed in the same, repeated, periodic manner along each series. The periodical table of the elements, as first proposed by Mendeleev, was completed in the following years when the elements lacking were discovered, and their chemical and physical properties defined. Finally, when the electronic str ...
... changed in the same, repeated, periodic manner along each series. The periodical table of the elements, as first proposed by Mendeleev, was completed in the following years when the elements lacking were discovered, and their chemical and physical properties defined. Finally, when the electronic str ...
III. Periodic Trends
... Deduced elements existed, but were undiscovered elements, their properties could be predicted ...
... Deduced elements existed, but were undiscovered elements, their properties could be predicted ...
Groups
... (representative) elements, numbers ending in A, or #1-2 & 13-18. • Ten shorter groups of transition metals, numbers ending in B or #3-12. • Elements in the same group have the same valence electron configuration, and therefore have similar chemical and ...
... (representative) elements, numbers ending in A, or #1-2 & 13-18. • Ten shorter groups of transition metals, numbers ending in B or #3-12. • Elements in the same group have the same valence electron configuration, and therefore have similar chemical and ...
Lesson 7.8 Basic Properties of the Main Group Elements Suggested
... The Group IA and Group IIA elements are all relatively reactive metals, and there is only a slight variation in metallic character. Within the group IIIA elements, we see a significant increase in metallic character going down the column of elements. The first Group IIIA element, boron, is a metall ...
... The Group IA and Group IIA elements are all relatively reactive metals, and there is only a slight variation in metallic character. Within the group IIIA elements, we see a significant increase in metallic character going down the column of elements. The first Group IIIA element, boron, is a metall ...
- Williamsburg High School for
... • In the ground state, each atom of an element has two valence ...
... • In the ground state, each atom of an element has two valence ...
Period Trend
... Of the elements magnesium, Mg, chlorine, Cl, sodium, Na, and phosphorus, P, which has the largest atomic radius? Explain your answer in terms of trends in the periodic table. 1. Find the elements listed in the periodic table. 2. Put the elements in order and determine the trend. ...
... Of the elements magnesium, Mg, chlorine, Cl, sodium, Na, and phosphorus, P, which has the largest atomic radius? Explain your answer in terms of trends in the periodic table. 1. Find the elements listed in the periodic table. 2. Put the elements in order and determine the trend. ...
Periodic Properties
... Atomic Size - the size of the atom decreases as you move from the left side of the chart to the right side of the chart; the size of the atom increases as you move down a column in the chart a. adding additional orbitals causes the size of the atom to increase as you move down the chart b. adding ad ...
... Atomic Size - the size of the atom decreases as you move from the left side of the chart to the right side of the chart; the size of the atom increases as you move down a column in the chart a. adding additional orbitals causes the size of the atom to increase as you move down the chart b. adding ad ...
GENERAL CHARACTERISTICS OF THE p
... observed in the properties of these compounds on moving down any particular group. The noble gases have almost zero electron afinity and have very high ionization enthalpies. Therefore, under normal conditions, the atoms of noble gases have little tendency to gain ...
... observed in the properties of these compounds on moving down any particular group. The noble gases have almost zero electron afinity and have very high ionization enthalpies. Therefore, under normal conditions, the atoms of noble gases have little tendency to gain ...
Periodic Table 2015
... • Dmitri Mendeleev Arranged elements by similar properties but left blanks for undiscovered elements ...
... • Dmitri Mendeleev Arranged elements by similar properties but left blanks for undiscovered elements ...
Periodic Law
... The pattern of properties within a period repeats as you move from one period to the next. ...
... The pattern of properties within a period repeats as you move from one period to the next. ...
Group 16: The Oxygen Family - Chemwiki
... Tellurium is the metalloid of the oxygen family, with a silvery white color and a metallic luster similar to that of tin at room temperature. Like selenium, it is also displays photoconductivity. Tellurium is an extremely rare element, and is most commonly found as a telluride of gold. It is often u ...
... Tellurium is the metalloid of the oxygen family, with a silvery white color and a metallic luster similar to that of tin at room temperature. Like selenium, it is also displays photoconductivity. Tellurium is an extremely rare element, and is most commonly found as a telluride of gold. It is often u ...
Ch. 6 PPT
... Ionic Size - change in the size (radius) of an atom when it gains or loses electrons Ion = An atom that has gained or lost an electron and now has a net charge (positive or negative) KC 17 : When at atom gains or loses an electron Cation = A positively charged atom that has lost one or more electro ...
... Ionic Size - change in the size (radius) of an atom when it gains or loses electrons Ion = An atom that has gained or lost an electron and now has a net charge (positive or negative) KC 17 : When at atom gains or loses an electron Cation = A positively charged atom that has lost one or more electro ...
Agenda 11/2/2016
... - Moving across a period the increased nuclear charge increases the pull of the nucleus on the electrons in the orbitals in that energy level - the outermost electrons are pulled closer to the nucleus and atomic radius gets smaller ...
... - Moving across a period the increased nuclear charge increases the pull of the nucleus on the electrons in the orbitals in that energy level - the outermost electrons are pulled closer to the nucleus and atomic radius gets smaller ...
b. - s3.amazonaws.com
... Strontium is an element that gives a brilliant red color to fireworks. a. In what group is strontium found? b. In what chemical family is strontium found? c. In what period is strontium found? d. What are the name and symbol of the element in Period 3 that is in the same group as strontium? e. What ...
... Strontium is an element that gives a brilliant red color to fireworks. a. In what group is strontium found? b. In what chemical family is strontium found? c. In what period is strontium found? d. What are the name and symbol of the element in Period 3 that is in the same group as strontium? e. What ...
CHAPTER-3 CLASSIFICATION OF ELEMENTS AND
... The carbonates of alkaline earth metals are relatively less stable. On heating, they decompose to give corresponding oxide and CO 2 gas. The decomposition temperature for alkaline earth metal carbonates increases as we go down the group. Anomalous Properties of Second Period Elements Their anomalous ...
... The carbonates of alkaline earth metals are relatively less stable. On heating, they decompose to give corresponding oxide and CO 2 gas. The decomposition temperature for alkaline earth metal carbonates increases as we go down the group. Anomalous Properties of Second Period Elements Their anomalous ...
Chemical Periodicity
... Low density, weak, brittle (non-malleable), poor conductor of heat. metalloids or semimetals High density, in between strong and weak, brittle, fair conductors of heat. 2 Who is Dmitri Mendeleev, and what was his contribution to chemistry? He was a chemist who worked on trying to figure out the ...
... Low density, weak, brittle (non-malleable), poor conductor of heat. metalloids or semimetals High density, in between strong and weak, brittle, fair conductors of heat. 2 Who is Dmitri Mendeleev, and what was his contribution to chemistry? He was a chemist who worked on trying to figure out the ...
Catalyst – September, 7(1+1) 2009 - stroh
... Why is this relationship true? Atoms with HIGH ELECTRONEGATIVITIES hold their electrons very close! Sooooo, the atomic radius decreases ...
... Why is this relationship true? Atoms with HIGH ELECTRONEGATIVITIES hold their electrons very close! Sooooo, the atomic radius decreases ...
TOC 9/18 Organization of Periodic Table
... Reactivity is a chemical property that determines how elements will react with others to form compounds. ...
... Reactivity is a chemical property that determines how elements will react with others to form compounds. ...
Record: 1 THE EVOLUTION OF THE PERIODIC SYSTEM Page 1 of
... Danish physicist Niels Bohr, the first to bring quantum theory to bear on the structure of the atom, was also motivated by the arrangement of the elements in the periodic system. In Bohr's model of the atom, developed in 1913, electrons inhabit a series of concentric shells that encircle the nucleus ...
... Danish physicist Niels Bohr, the first to bring quantum theory to bear on the structure of the atom, was also motivated by the arrangement of the elements in the periodic system. In Bohr's model of the atom, developed in 1913, electrons inhabit a series of concentric shells that encircle the nucleus ...
Metals
... Ch9.5 Naming Acids and Bases (Discuss these in more detail later) Acids: A compound that contains one or more hydrogen atoms and produces hydrogen ions (H+) when dissolved in water. (The names of compounds can change when acids are present). The name of an acidic compound depends on the type of a ...
... Ch9.5 Naming Acids and Bases (Discuss these in more detail later) Acids: A compound that contains one or more hydrogen atoms and produces hydrogen ions (H+) when dissolved in water. (The names of compounds can change when acids are present). The name of an acidic compound depends on the type of a ...
The Periodic Table!
... Very dense Can be found in smoke detectors, nuclear weapons, and radio active minerals ...
... Very dense Can be found in smoke detectors, nuclear weapons, and radio active minerals ...
Periodic Trends Reading
... top of Group 1, lithium is the least reactive, sodium is more reactive, and potassium is still more reactive. In other words, there is a trend toward great reactivity as you move down the alkali metals in Group 1. Understanding a trend among the elements allows you to make predictions about the chem ...
... top of Group 1, lithium is the least reactive, sodium is more reactive, and potassium is still more reactive. In other words, there is a trend toward great reactivity as you move down the alkali metals in Group 1. Understanding a trend among the elements allows you to make predictions about the chem ...
Periodic Table 1
... In an isolated atom all of the d sublevel electrons have the same energy. When an atom is surrounded by charged ions or polar molecules, the electric field from these ions or molecules has a unequal effect on the energies of the various d orbitals and d electrons. The colors of the ions and complex ...
... In an isolated atom all of the d sublevel electrons have the same energy. When an atom is surrounded by charged ions or polar molecules, the electric field from these ions or molecules has a unequal effect on the energies of the various d orbitals and d electrons. The colors of the ions and complex ...
The Nuts and Bolts of Periodic Tables
... Elements can be arranged the same way. In the late 1860's DMITRI MENDELEEV organized all of the 63 known elements into a table. In this periodic table he arrange elements based on atomic, physical and chemical properties. There were some gaps in his table and he correctly predicted the existence of ...
... Elements can be arranged the same way. In the late 1860's DMITRI MENDELEEV organized all of the 63 known elements into a table. In this periodic table he arrange elements based on atomic, physical and chemical properties. There were some gaps in his table and he correctly predicted the existence of ...