Chapter 2 Elements are made up of small particles called atoms. All
... The periodic table is a chart where elements are organized depending on how similar they are. The horizontal rows are called periods and the vertical columns are called groups or families. ...
... The periodic table is a chart where elements are organized depending on how similar they are. The horizontal rows are called periods and the vertical columns are called groups or families. ...
Chapter 3 Section 2 Notes
... number of protons and electrons increases by one Elements in the same period DO NOT have similar properties; in fact, they change greatly across the row The first element in a period is always an extremely active solid. The last element in a period, is always an inactive gas. ...
... number of protons and electrons increases by one Elements in the same period DO NOT have similar properties; in fact, they change greatly across the row The first element in a period is always an extremely active solid. The last element in a period, is always an inactive gas. ...
In modern periodic table, elements in the same column have similar
... column, gaps had to be left in his table. • He predicted elements would be discovered to fill the gaps • Also correctly predicted properties of these undiscovered elements ...
... column, gaps had to be left in his table. • He predicted elements would be discovered to fill the gaps • Also correctly predicted properties of these undiscovered elements ...
graphing atomic properties
... 6. Circle the atom that would require the LEAST amount of energy to remove an ea. magnesium, chlorine, silicon b. lithium, cesium, potassium c. fluorine, iodine, chlorine d. calcium, bromine, cobalt 7. Circle the atom that would require the MOST amount of energy to remove an ea. lithium, potassium, ...
... 6. Circle the atom that would require the LEAST amount of energy to remove an ea. magnesium, chlorine, silicon b. lithium, cesium, potassium c. fluorine, iodine, chlorine d. calcium, bromine, cobalt 7. Circle the atom that would require the MOST amount of energy to remove an ea. lithium, potassium, ...
The Periodic Table and Trends of the Elements
... The inner transition metals from 58-71 are the Lanthanide. They are a part of period 6. They are very large atoms. The inner transition metals from 90-103 are the Actinides. They are part of period 7. All of the elements occurring after Uranium are man made and most exist only for a short time ...
... The inner transition metals from 58-71 are the Lanthanide. They are a part of period 6. They are very large atoms. The inner transition metals from 90-103 are the Actinides. They are part of period 7. All of the elements occurring after Uranium are man made and most exist only for a short time ...
periodic table power point
... The inner transition metals from 58-71 are the Lanthanide. They are a part of period 6. They are very large atoms. The inner transition metals from 90-103 are the Actinides. They are part of period 7. All of the elements occurring after Uranium are man made and most exist only for a short time ...
... The inner transition metals from 58-71 are the Lanthanide. They are a part of period 6. They are very large atoms. The inner transition metals from 90-103 are the Actinides. They are part of period 7. All of the elements occurring after Uranium are man made and most exist only for a short time ...
20161025131513
... o what patterns did he noticeo what was his final arrangement of the periodic tableo what was missing in his tableo how did he predict undiscovered elementso was he the first to make a periodic tableo what does the placement of the elements reveal links betweeno how were his predictionsSection 2 V ...
... o what patterns did he noticeo what was his final arrangement of the periodic tableo what was missing in his tableo how did he predict undiscovered elementso was he the first to make a periodic tableo what does the placement of the elements reveal links betweeno how were his predictionsSection 2 V ...
Unit 4 - The Periodic Table
... He, Ne and Ar are much higher on the graph because it takes more energy to remove an electron from these atoms (noble gasses are very stable). Li, Na and K are much lower on the graph because they have a single valence electron that is easier to remove. ...
... He, Ne and Ar are much higher on the graph because it takes more energy to remove an electron from these atoms (noble gasses are very stable). Li, Na and K are much lower on the graph because they have a single valence electron that is easier to remove. ...
notes - unit 3 - periodic table_student_2014
... Prefer to ________ their two electrons to become ___ ions ___________ reactive never found alone in nature Groups 3-12 TRANSITION METALS Found in the ___________ of the periodic table (the D block) Form ___________________ in solution (ex: Cu is bright blue when dissolved in water) This ...
... Prefer to ________ their two electrons to become ___ ions ___________ reactive never found alone in nature Groups 3-12 TRANSITION METALS Found in the ___________ of the periodic table (the D block) Form ___________________ in solution (ex: Cu is bright blue when dissolved in water) This ...
Physical Science 100 Chapter 18, The Periodic Table
... Metals and non-metals are separated by a red “staircase” Elements near this staircase are called semi-metals (or semi-conductors) The noble or inert gases are on the far right. ...
... Metals and non-metals are separated by a red “staircase” Elements near this staircase are called semi-metals (or semi-conductors) The noble or inert gases are on the far right. ...
Chapter 2 - Test Bank
... mass of the other element are in ratios of small whole numbers. As an example, chlorine will combine with phosphorous to form PCl3 and PCl5. The law of multiple proportions suggests that for a given mass of phosphorous (e.g., 30.97 g, the mass of one mole of phosphorus), the ratio of masses of chlor ...
... mass of the other element are in ratios of small whole numbers. As an example, chlorine will combine with phosphorous to form PCl3 and PCl5. The law of multiple proportions suggests that for a given mass of phosphorous (e.g., 30.97 g, the mass of one mole of phosphorus), the ratio of masses of chlor ...
File
... • Only valence electrons are shown. • Dots representing valence electrons are placed around the element symbols (on 4 sides, imagine a box around the symbol). • Electron dots are placed singularly, then they are paired. ...
... • Only valence electrons are shown. • Dots representing valence electrons are placed around the element symbols (on 4 sides, imagine a box around the symbol). • Electron dots are placed singularly, then they are paired. ...
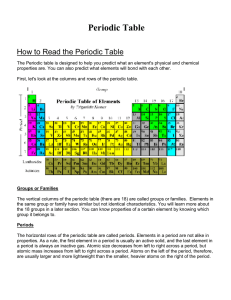
How to Read the Periodic Table
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
Periodic Table
... protons and electrons • Today we know that different isotopes of the same element have the same properties, but have different masses because of neutrons which affect the mass, but not the properties. ...
... protons and electrons • Today we know that different isotopes of the same element have the same properties, but have different masses because of neutrons which affect the mass, but not the properties. ...
Periodic Trends Name: Part 1: Summary of the Periodic Trends
... b. How does this account for the trend you discovered in 1st ionization energy? ...
... b. How does this account for the trend you discovered in 1st ionization energy? ...
The Periodic Table
... A measure of the ability of an atom in a chemical compound to attract electrons Electronegativities tend to increase across a period Electronegativities tend to decrease down a group or remain the same ...
... A measure of the ability of an atom in a chemical compound to attract electrons Electronegativities tend to increase across a period Electronegativities tend to decrease down a group or remain the same ...
Chapter 5 Organizing The Elements
... • Solids, liquids, gases based on their state at room temperature • Symbols solids (black) liquid (purple) gas (red) • Elements are divided into those that occur naturally and those that do not • Ex. Elements with an atomic number of 93 or above DO NOT OCCUR NATURALLY • 1-92 occur naturally ...
... • Solids, liquids, gases based on their state at room temperature • Symbols solids (black) liquid (purple) gas (red) • Elements are divided into those that occur naturally and those that do not • Ex. Elements with an atomic number of 93 or above DO NOT OCCUR NATURALLY • 1-92 occur naturally ...
Ex. 06 Answer
... Across a period, the elements show a gradual change (decrease) in atomic size. b) 5 c) 2 d) Metals — a, b, c, d Metalloid — e Non-metals — f, g, h, i, j ...
... Across a period, the elements show a gradual change (decrease) in atomic size. b) 5 c) 2 d) Metals — a, b, c, d Metalloid — e Non-metals — f, g, h, i, j ...
Part D Questions and Problems
... 2. a. nonmetal d. nonmetal b. metalloid e. metal c. metal 3. a 4. Li, Na, Rb, Cs, Fr 5. The three classes are as follows. 1) The metals: good conductors of heat and electric current; high luster when clean; malleable; ductile. 2) The nonmetals: poor conductors of heat and electric current; nonlustro ...
... 2. a. nonmetal d. nonmetal b. metalloid e. metal c. metal 3. a 4. Li, Na, Rb, Cs, Fr 5. The three classes are as follows. 1) The metals: good conductors of heat and electric current; high luster when clean; malleable; ductile. 2) The nonmetals: poor conductors of heat and electric current; nonlustro ...
REVIEW Through Course Task
... 10. While metals come in many colors, most at the far left side of the periodic table are very ____________ in color and tend to become more _________ or darker colored toward the center SILVER DULL and right side of the table. 11. While, as a general rule, most metals have high melting points, ther ...
... 10. While metals come in many colors, most at the far left side of the periodic table are very ____________ in color and tend to become more _________ or darker colored toward the center SILVER DULL and right side of the table. 11. While, as a general rule, most metals have high melting points, ther ...
The History of the Modern Periodic Table
... effect” caused by the addition of new energy levels. The inner energy levels act in a way to shield the attractive charges of the nucleus for the outer electrons. ...
... effect” caused by the addition of new energy levels. The inner energy levels act in a way to shield the attractive charges of the nucleus for the outer electrons. ...
Periodicity Review - Dr. Antony`s Chem Help
... Elements to the left of the stair step line are metals, and therefore are easily oxidized (lose electrons) in bonding situations, are good electrical conductors, are shiny, malleable, ductile, and have low ionization energies and electronegativities. Elements to the right of the stair step line, ...
... Elements to the left of the stair step line are metals, and therefore are easily oxidized (lose electrons) in bonding situations, are good electrical conductors, are shiny, malleable, ductile, and have low ionization energies and electronegativities. Elements to the right of the stair step line, ...
Periodic table of elements
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
Chapter Test A
... c. Na d. Fr ______19. For atoms of p-block elements, the total number of electrons in the highest occupied level is equal to the a. period number. b. group number. c. period number minus 10. d. group number minus 10. ______20. As electrons add to s and p sublevels in the same main energy level, they ...
... c. Na d. Fr ______19. For atoms of p-block elements, the total number of electrons in the highest occupied level is equal to the a. period number. b. group number. c. period number minus 10. d. group number minus 10. ______20. As electrons add to s and p sublevels in the same main energy level, they ...