The History of the Modern Periodic Table
... Periodic Table • Periodic Table: arrangement of elements in order of their atomic numbers so that elements with similar properties fall in the same column. ...
... Periodic Table • Periodic Table: arrangement of elements in order of their atomic numbers so that elements with similar properties fall in the same column. ...
Periodic Table Worksheet
... 16. As you go from left to right across the periodic table, the elements go from (METALS / nonmetals) to (metals / NONMETALS). 17. The most active element in Group 17 is FLUORINE. 18. What sublevels are filling across the Transition Elements? d AND f 19. Elements within a group have a similar number ...
... 16. As you go from left to right across the periodic table, the elements go from (METALS / nonmetals) to (metals / NONMETALS). 17. The most active element in Group 17 is FLUORINE. 18. What sublevels are filling across the Transition Elements? d AND f 19. Elements within a group have a similar number ...
Periodic Table Funsheet (KEY) 1. Where are the most active metals
... 16. As you go from left to right across the periodic table, the elements go from (METALS / nonmetals) to (metals / NONMETALS). 17. The most active element in Group 17 is FLUORINE. 18. What sublevels are filling across the Transition Elements? d AND f 19. Elements within a group have a similar number ...
... 16. As you go from left to right across the periodic table, the elements go from (METALS / nonmetals) to (metals / NONMETALS). 17. The most active element in Group 17 is FLUORINE. 18. What sublevels are filling across the Transition Elements? d AND f 19. Elements within a group have a similar number ...
Test 1. 2nd prep. ques
... 3- By increasing the atomic number of element in group 1A the metallic property increases. because the atomic size increases. 4- Cobalt 60 is used in preservation of food. because it emits gamma rays which stop the reproduction of microbial cells5- Both sodium ion (Na+) and fluoride ion (F-) have th ...
... 3- By increasing the atomic number of element in group 1A the metallic property increases. because the atomic size increases. 4- Cobalt 60 is used in preservation of food. because it emits gamma rays which stop the reproduction of microbial cells5- Both sodium ion (Na+) and fluoride ion (F-) have th ...
The Periodic Table
... So how is it arranged? The periodic table is organized in a grid. The elements are placed in specific places because of the way they look and act. There are rows (left to right) and columns (up and down) , and they each mean ...
... So how is it arranged? The periodic table is organized in a grid. The elements are placed in specific places because of the way they look and act. There are rows (left to right) and columns (up and down) , and they each mean ...
Chemical Periodicity
... • Addison-Wesley Chemistry, Chapter 12 Chemical Periodicity • Merrill Chemistry A Modern Course, Chapter 9 Periodic Table • Heath Chemistry: Experiments and Principles, Chapter 7 Order Among Atomsdels of Atomic ...
... • Addison-Wesley Chemistry, Chapter 12 Chemical Periodicity • Merrill Chemistry A Modern Course, Chapter 9 Periodic Table • Heath Chemistry: Experiments and Principles, Chapter 7 Order Among Atomsdels of Atomic ...
File - CCHS Chemistry
... elems w/ similar props in the same column Thought elems had yet to be discovered Predicted props & atomic masses of several elems Eventually discovered & his predictions were very close ...
... elems w/ similar props in the same column Thought elems had yet to be discovered Predicted props & atomic masses of several elems Eventually discovered & his predictions were very close ...
File
... Vertical columns in atomic mass order Made some exceptions to place elements in rows with similar properties (Tellurium and Iodine) Horizontal rows have similar chemical properties Gaps for “yet to be discovered” elements Left questions: why didn’t some elements fit in order of increasing mass? Why ...
... Vertical columns in atomic mass order Made some exceptions to place elements in rows with similar properties (Tellurium and Iodine) Horizontal rows have similar chemical properties Gaps for “yet to be discovered” elements Left questions: why didn’t some elements fit in order of increasing mass? Why ...
6-Getting to Know the Periodic Table
... Assignment 1: Getting to Know the Periodic Table Complete the following objectives using the periodic table below. ...
... Assignment 1: Getting to Know the Periodic Table Complete the following objectives using the periodic table below. ...
The Periodic Table
... Silicon is brittle like a nonmetal Silicon is a semiconductor of electricity ...
... Silicon is brittle like a nonmetal Silicon is a semiconductor of electricity ...
Develo ment of the Atomic Theo John Dalton - (English 1766
... elements appeared to be misplaced in terms of their properties. He assumed that their atomic mass was just calculated incorrectly. 50 yrs. later a British scientist - Henry Moseley discovered the atomic numbers of the elements. He noticed that when the elements were rearranged in order of increasing ...
... elements appeared to be misplaced in terms of their properties. He assumed that their atomic mass was just calculated incorrectly. 50 yrs. later a British scientist - Henry Moseley discovered the atomic numbers of the elements. He noticed that when the elements were rearranged in order of increasing ...
bg`d xng gmz moxa gmog dbcxd gmz tovd gmog
... 2. Describe the modern periodic table 3. Explain how the periodic law can be used to predict the physical and chemical properties of elements. 4. Describe how the elements belonging to a group of the periodic table are interrelated in terms of atomic number. By 1860, more than 60 elements were known ...
... 2. Describe the modern periodic table 3. Explain how the periodic law can be used to predict the physical and chemical properties of elements. 4. Describe how the elements belonging to a group of the periodic table are interrelated in terms of atomic number. By 1860, more than 60 elements were known ...
AP Chemistry Chapter 7
... When Moseley arranged the elements of Mendeleev’s periodic table according to increasing atomic number and not atomic mass, the inconsistencies associated with Mendeleev's table were eliminated. The modern periodic table is based on Moseley's arrangement by atomic number. At age 28, Moseley was kill ...
... When Moseley arranged the elements of Mendeleev’s periodic table according to increasing atomic number and not atomic mass, the inconsistencies associated with Mendeleev's table were eliminated. The modern periodic table is based on Moseley's arrangement by atomic number. At age 28, Moseley was kill ...
Periodic Table and Trends
... Organization of the Periodic Table First created by Dmitri Mendeleev ...
... Organization of the Periodic Table First created by Dmitri Mendeleev ...
Periodic Table Notes
... the table, atoms tend to become smaller in size, but ___________ in mass. Which way do the periods go?____________________________________ ...
... the table, atoms tend to become smaller in size, but ___________ in mass. Which way do the periods go?____________________________________ ...
clean-color-coded-periodic-table_ochoa-edit
... TURN IT IN BEFORE YOU LEAVE! Color-Coded Periodic Table Activity Directions Directions: Be sure to follow all instructions carefully and completely! Use your textbook (the back cover and pp. 177-181 will be very useful!) and any other resources to help you complete the periodic table. 1. Draw a RED ...
... TURN IT IN BEFORE YOU LEAVE! Color-Coded Periodic Table Activity Directions Directions: Be sure to follow all instructions carefully and completely! Use your textbook (the back cover and pp. 177-181 will be very useful!) and any other resources to help you complete the periodic table. 1. Draw a RED ...
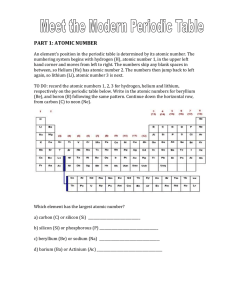
PART 1: ATOMIC NUMBER - hrsbstaff.ednet.ns.ca
... An element’s position in the periodic table is determined by its atomic number. The numbering system begins with hydrogen (H), atomic number 1, in the upper left hand corner and moves from left to right. The numbers skip any blank spaces in between, so Helium (He) has atomic number 2. The numbers th ...
... An element’s position in the periodic table is determined by its atomic number. The numbering system begins with hydrogen (H), atomic number 1, in the upper left hand corner and moves from left to right. The numbers skip any blank spaces in between, so Helium (He) has atomic number 2. The numbers th ...
Chemistry Study Cards Chapter 5 (3-2) The length of each period in
... When chemical compounds form, lost, gained, or shared. valence electrons are those that may be The number of valence electrons in Group 1 elements is ...
... When chemical compounds form, lost, gained, or shared. valence electrons are those that may be The number of valence electrons in Group 1 elements is ...
Topic 5 - Holy Cross Collegiate
... Mendeleev left gaps in his table, blank spaces predicting the existence of elements not yet found or even suspected by other chemists. He even predicted properties of these unknown elements, which spurred on other scientists to prove or disprove his predictions. How did Mendeleev’s table make it pos ...
... Mendeleev left gaps in his table, blank spaces predicting the existence of elements not yet found or even suspected by other chemists. He even predicted properties of these unknown elements, which spurred on other scientists to prove or disprove his predictions. How did Mendeleev’s table make it pos ...
File
... Mendeleev left gaps in his table, blank spaces predicting the existence of elements not yet found or even suspected by other chemists. He even predicted properties of these unknown elements, which spurred on other scientists to prove or disprove his predictions. How did Mendeleev’s table make it pos ...
... Mendeleev left gaps in his table, blank spaces predicting the existence of elements not yet found or even suspected by other chemists. He even predicted properties of these unknown elements, which spurred on other scientists to prove or disprove his predictions. How did Mendeleev’s table make it pos ...
Unit 2 Overview
... Classify elements as metals, nonmetals, or metalloids using the Periodic Table. List general properties of metal, nonmetal, and metalloid elements. Research & present specific properties about an individual element. ...
... Classify elements as metals, nonmetals, or metalloids using the Periodic Table. List general properties of metal, nonmetal, and metalloid elements. Research & present specific properties about an individual element. ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.