Chapter 5 Chem classnotes
... from a neutral atom of an element. As atomic number increases going down a group, more electrons lie between the nucleus and the electrons in the outer orbits. This shields the outer electrons from the nuclear forces of attraction. IE increases left to right (across a period) and decrease top to bot ...
... from a neutral atom of an element. As atomic number increases going down a group, more electrons lie between the nucleus and the electrons in the outer orbits. This shields the outer electrons from the nuclear forces of attraction. IE increases left to right (across a period) and decrease top to bot ...
Introducing the Elements - Paul M. Dorman High School
... • Most tables at the time listed elements by mass • Mendeleev also arranged elements by mass, but left several “holes” in his table and occasionally reversed the order of elements to fit the properties of others in that column • The “holes” were later filled in with newly discovered elements that ha ...
... • Most tables at the time listed elements by mass • Mendeleev also arranged elements by mass, but left several “holes” in his table and occasionally reversed the order of elements to fit the properties of others in that column • The “holes” were later filled in with newly discovered elements that ha ...
The Periodic Table
... group have similar chemical and physical properties!! (Mendeleev did that on purpose.) • Elements in the”A” groups are called representative elements. ...
... group have similar chemical and physical properties!! (Mendeleev did that on purpose.) • Elements in the”A” groups are called representative elements. ...
The Periodic Table - Anderson High School
... group have similar chemical and physical properties!! (Mendeleev did that on purpose.) • Elements in the”A” groups are called representative elements. ...
... group have similar chemical and physical properties!! (Mendeleev did that on purpose.) • Elements in the”A” groups are called representative elements. ...
Chapter 6 notes
... 6.1 Organizing the Elements Searching for an organizing principle Chemists used the properties of elements to sort them into groups Mendeleev's Periodic Table In ___________, a Russian chemist and teacher, Dmitri Mendeleev, published a table of the elements. Later that year, a German chemist, Lothar ...
... 6.1 Organizing the Elements Searching for an organizing principle Chemists used the properties of elements to sort them into groups Mendeleev's Periodic Table In ___________, a Russian chemist and teacher, Dmitri Mendeleev, published a table of the elements. Later that year, a German chemist, Lothar ...
orbital form the s block (groups 1 and 2). Elements in
... increasing atomic number. The vertical columns in the periodic table are referred to as groups and the horizontal rows are known as periods. The elements in groups 1 and 2 and those in groups 13 to 18 are called main-group elements. Elements in groups 3 to 12 are known as transition elements and the ...
... increasing atomic number. The vertical columns in the periodic table are referred to as groups and the horizontal rows are known as periods. The elements in groups 1 and 2 and those in groups 13 to 18 are called main-group elements. Elements in groups 3 to 12 are known as transition elements and the ...
The Periodic Law
... order of their atomic numbers so that elements with similar properties fall in the same column or group. • The most significant additions were the noble gases. Helium was discovered in 1868. Argon was discovered by Ramsey in 1894. He also discovered krypton and xenon in 1898. Dorn discovered radon i ...
... order of their atomic numbers so that elements with similar properties fall in the same column or group. • The most significant additions were the noble gases. Helium was discovered in 1868. Argon was discovered by Ramsey in 1894. He also discovered krypton and xenon in 1898. Dorn discovered radon i ...
File - Lenora Henderson`s Flipped Chemistry Classroom
... Reading the Periodic Table Key Question What information can be displayed in a periodic table? The symbols and names of the elements, along with information about the structure of their atoms. Group 1A elements are called alkali metals Group 2A elements are called alkaline-earth metals Group ...
... Reading the Periodic Table Key Question What information can be displayed in a periodic table? The symbols and names of the elements, along with information about the structure of their atoms. Group 1A elements are called alkali metals Group 2A elements are called alkaline-earth metals Group ...
Periodic Classification of Elements
... It was Henry Moseley who demonstrated that atomic number of an element could explain periodic properties in a better way than atomic mass of an element and arranged the elements in increasing order of their atomic numbers. Then it was found that the various anomalies of Mendeleev’s periodic table we ...
... It was Henry Moseley who demonstrated that atomic number of an element could explain periodic properties in a better way than atomic mass of an element and arranged the elements in increasing order of their atomic numbers. Then it was found that the various anomalies of Mendeleev’s periodic table we ...
Periodic Table - Red Deer Public
... ANIONS are LARGER than the atoms from which they come. The electron/proton attraction has gone DOWN (core charge decreases) and so ...
... ANIONS are LARGER than the atoms from which they come. The electron/proton attraction has gone DOWN (core charge decreases) and so ...
The Periodic Table of the Elements
... elements were organized by increasing atomic mass, certain similarities in their chemical properties occurred at regular intervals. Such repeating patterns are referred to as PERIODIC. • Mendeleev created a table in which elements with similar properties were grouped together—the first Periodic Tabl ...
... elements were organized by increasing atomic mass, certain similarities in their chemical properties occurred at regular intervals. Such repeating patterns are referred to as PERIODIC. • Mendeleev created a table in which elements with similar properties were grouped together—the first Periodic Tabl ...
Periodic Table Notes 2
... in a meaningful way. He developed classification scheme based upon molar masses. Elements that showed similar patterns of chemical behavior were placed into groups. His chart was incomplete at the time, but Mendeleev predicted that new elements were yet to be discovered, so he left blank spaces in h ...
... in a meaningful way. He developed classification scheme based upon molar masses. Elements that showed similar patterns of chemical behavior were placed into groups. His chart was incomplete at the time, but Mendeleev predicted that new elements were yet to be discovered, so he left blank spaces in h ...
File
... upon their similar properties. He left blank spaces where he thought undiscovered elements would go, based on their properties ...
... upon their similar properties. He left blank spaces where he thought undiscovered elements would go, based on their properties ...
Periodic Table - Mendeleev (1869): --
... - since there are no "molecules", an ionic formula cannot describe how many and what kinds of atoms are in a molecule! - all ionic compounds are observed to be (overall) electrically neutral, so the IONS they contain must be present in such a way that the charges BALANCE EACH OTHER - an ionic formul ...
... - since there are no "molecules", an ionic formula cannot describe how many and what kinds of atoms are in a molecule! - all ionic compounds are observed to be (overall) electrically neutral, so the IONS they contain must be present in such a way that the charges BALANCE EACH OTHER - an ionic formul ...
Science 2nd prep 1st term 1st lesson Many attempts are made by
... *The number of known elements until now are 116 elements, 92 elements are available in the earth’s crust, the rest of the elements are prepared artificially. *Elements of (A) groups lie on the left and right of the table, you can locate their position in the modern periodic table by knowing their at ...
... *The number of known elements until now are 116 elements, 92 elements are available in the earth’s crust, the rest of the elements are prepared artificially. *Elements of (A) groups lie on the left and right of the table, you can locate their position in the modern periodic table by knowing their at ...
Port Said International Schools
... 5. The element which locates in period 3 and group 3A is (13Al – 5B– 11Na – 15P). 6. Which of the following elements locates in the third period (19K – 15P – 6C– 3Li). 7. All the following elements are located in group (2A) except (4Be - 20Ca- 11Na – 12Mg). 8. By increasing the atomic number within ...
... 5. The element which locates in period 3 and group 3A is (13Al – 5B– 11Na – 15P). 6. Which of the following elements locates in the third period (19K – 15P – 6C– 3Li). 7. All the following elements are located in group (2A) except (4Be - 20Ca- 11Na – 12Mg). 8. By increasing the atomic number within ...
specific vocabulary of the unit
... expressed in atomic mass units. Atomic mass unit /ə'tɒmɪk//mæs//'ju:nɪt/ ...
... expressed in atomic mass units. Atomic mass unit /ə'tɒmɪk//mæs//'ju:nɪt/ ...
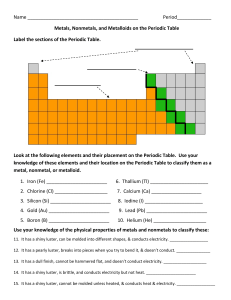
Name Period_____________ Metals, Nonmetals, and Metalloids on
... 13. It has a dull finish, cannot be hammered flat, and doesn’t conduct electricity. ___________________ 14. It has a shiny luster, is brittle, and conducts electricity but not heat. ______________________ 15. It has a shiny luster, cannot be molded unless heated, & conducts heat & electricity. _____ ...
... 13. It has a dull finish, cannot be hammered flat, and doesn’t conduct electricity. ___________________ 14. It has a shiny luster, is brittle, and conducts electricity but not heat. ______________________ 15. It has a shiny luster, cannot be molded unless heated, & conducts heat & electricity. _____ ...
Revision map for the Periodic Table
... You are asked to think up sentences to show how the different words in the boxes are related. Try and be as accurate and precise as you can. Make sure you put each sentence next to the correct number in the spaces below. One of the sentences has been suggested for you, to get you ...
... You are asked to think up sentences to show how the different words in the boxes are related. Try and be as accurate and precise as you can. Make sure you put each sentence next to the correct number in the spaces below. One of the sentences has been suggested for you, to get you ...
word - My eCoach
... energy. Plot the ionization energy (y-axis) of the first 54 elements against their atomic number (x-axis). 2. Label each point on the graph with the symbol of the element. Analysis and Conclusion 1. Describe the general shape of the graph. ...
... energy. Plot the ionization energy (y-axis) of the first 54 elements against their atomic number (x-axis). 2. Label each point on the graph with the symbol of the element. Analysis and Conclusion 1. Describe the general shape of the graph. ...
File
... not reflect light. Non-metallic elements are very brittle and cannot be rolled into wires or pounded into sheets. They can either be gases or solids at room temperature. The elements include: Hydrogen, Carbon, Nitrogen, Oxygen, Phosphorus, Sulfur, and Selenium. Pick a highlighter and color all of th ...
... not reflect light. Non-metallic elements are very brittle and cannot be rolled into wires or pounded into sheets. They can either be gases or solids at room temperature. The elements include: Hydrogen, Carbon, Nitrogen, Oxygen, Phosphorus, Sulfur, and Selenium. Pick a highlighter and color all of th ...
Chapter 7.1 Notes
... Mendeleev and his periodic table. • The time is the 1860’s. Mendeleev is working on figuring out patterns in the physical and chemical properties of the known elements of the time. • Mendeleev used rows of elements to show similar chemical properties. If the elements were in the same row, they had ...
... Mendeleev and his periodic table. • The time is the 1860’s. Mendeleev is working on figuring out patterns in the physical and chemical properties of the known elements of the time. • Mendeleev used rows of elements to show similar chemical properties. If the elements were in the same row, they had ...
Definition - kcpe-kcse
... - elements with properties that fall between those of metals and non metals - chemical properties will vary, usually most like the region they are closer to ex. As: closer to non metal most of it’s property will resemble that ...
... - elements with properties that fall between those of metals and non metals - chemical properties will vary, usually most like the region they are closer to ex. As: closer to non metal most of it’s property will resemble that ...
Grouping of Elements in the Periodic Table
... 6. Which of the following statements is true? a) Some transactinides are also actinides. b) Some actinides are also lanthanides. c) Some transuraniums are also transactinides. d) Some lanthanides are also transuraniums. 7. Which elements are most likely to lose electrons and form cations? a) transit ...
... 6. Which of the following statements is true? a) Some transactinides are also actinides. b) Some actinides are also lanthanides. c) Some transuraniums are also transactinides. d) Some lanthanides are also transuraniums. 7. Which elements are most likely to lose electrons and form cations? a) transit ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.