2- methoxyaniline
... surface allows to restore partially the size of coefficient of reflection, and use, in addition, effect of laser cleaning ― to improve quality of mirrors in comparison with just polished mirror (R = 0,985). This method can be also used for cleaning of rather expensive products from non-ferrous metal ...
... surface allows to restore partially the size of coefficient of reflection, and use, in addition, effect of laser cleaning ― to improve quality of mirrors in comparison with just polished mirror (R = 0,985). This method can be also used for cleaning of rather expensive products from non-ferrous metal ...
Name
... P. Arrangement of elements in order of their atomic number so that elements with similar properties fall in the same column ...
... P. Arrangement of elements in order of their atomic number so that elements with similar properties fall in the same column ...
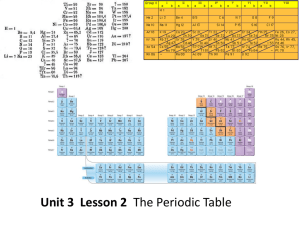
12.3 The Periodic Table
... Symbol and atomic mass: For each of the following, write the element name that corresponds to the symbol. In addition, write the atomic mass for each element. ...
... Symbol and atomic mass: For each of the following, write the element name that corresponds to the symbol. In addition, write the atomic mass for each element. ...
Unit 1 Learning Outcomes
... • explain why some elements are familiar, and others are not • explain why some elements don't occur naturally, and which ones these are • match groups of elements with how they were discovered • give examples of how elements were named • using the Periodic Table, give names and symbols of the eleme ...
... • explain why some elements are familiar, and others are not • explain why some elements don't occur naturally, and which ones these are • match groups of elements with how they were discovered • give examples of how elements were named • using the Periodic Table, give names and symbols of the eleme ...
Periodic Table Quiz
... The pictures below show the position of di erent elements on the periodic table. Which picture has an X in the locations of the three elements that would be most similar in the way they react? A. ...
... The pictures below show the position of di erent elements on the periodic table. Which picture has an X in the locations of the three elements that would be most similar in the way they react? A. ...
WS #10 - Atomic Theory and Periodic Table
... produced the forerunner to the modern weight Periodic Table. He arranged the elements in increasing atomic _______________ and placed properties elements having similar _______________ under one another to obtain a table. An important aspect of Mendeleev’s table was the fact that he recognised there ...
... produced the forerunner to the modern weight Periodic Table. He arranged the elements in increasing atomic _______________ and placed properties elements having similar _______________ under one another to obtain a table. An important aspect of Mendeleev’s table was the fact that he recognised there ...
The Periodic Table - Ms. Dormer
... PERIODIC TRENDS Predictable change in a particular direction Reactivity of Alkali metals ...
... PERIODIC TRENDS Predictable change in a particular direction Reactivity of Alkali metals ...
AP Chemistry Chapter 7
... order of increasing mass? Why did some elements exhibit periodic behavior? ...
... order of increasing mass? Why did some elements exhibit periodic behavior? ...
Periodic Table and Trends
... Organization of the Periodic Table First created by Dmitri Mendeleev ...
... Organization of the Periodic Table First created by Dmitri Mendeleev ...
periodic table
... • It was once believed by philosophers such as Aristotle, that fire, wind, earth, and water, in various combinations, made up all objects. • By the 1860s, scientists considered there to be at least 60 different basic substances, or elements. • Scientists found that many of these elements have simila ...
... • It was once believed by philosophers such as Aristotle, that fire, wind, earth, and water, in various combinations, made up all objects. • By the 1860s, scientists considered there to be at least 60 different basic substances, or elements. • Scientists found that many of these elements have simila ...
Atomic Structure and Periodic Table Review Guide
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
File
... Step 5 – Symbol - Every element on the Periodic Table has a symbol to identify. This symbol is a type of shorthand to make it easier to identify. The symbol consists of a capital letter, and sometimes, a lower case letter. The symbol will never be more than two letters. Step 6 – Atomic Mass – Eleme ...
... Step 5 – Symbol - Every element on the Periodic Table has a symbol to identify. This symbol is a type of shorthand to make it easier to identify. The symbol consists of a capital letter, and sometimes, a lower case letter. The symbol will never be more than two letters. Step 6 – Atomic Mass – Eleme ...
Unit 5 – The Periodic Table
... How is the periodic table arranged? How does periodicity explain the chemical and physical properties of the elements? How can chemical and physical properties of the elements be predicted based on their positions in the periodic table? ...
... How is the periodic table arranged? How does periodicity explain the chemical and physical properties of the elements? How can chemical and physical properties of the elements be predicted based on their positions in the periodic table? ...
Periodic Table Properties Notes s1
... table are called groups or families. • Each group contains elements with similar chemical properties. • Every element within a group has the same number of valence electrons. • The number of valence electrons determines an element’s chemical ...
... table are called groups or families. • Each group contains elements with similar chemical properties. • Every element within a group has the same number of valence electrons. • The number of valence electrons determines an element’s chemical ...
Homework
... Themes on the Periodic Table Directions: Answer the following questions using the Periodic Table. 1. How many groups (families) are there in the Periodic Table? 2. How many elements are in your Periodic Table? 3. How many periods are there in your Periodic Table? 4. What is the basic theme of organ ...
... Themes on the Periodic Table Directions: Answer the following questions using the Periodic Table. 1. How many groups (families) are there in the Periodic Table? 2. How many elements are in your Periodic Table? 3. How many periods are there in your Periodic Table? 4. What is the basic theme of organ ...
Holt Modern Chemistry -
... o alkaline-earth metal -- one of the elements of Group 2 of the periodic table (beryllium, magnesium, calcium, strontium, barium, and radium) o transition element -- one of the metals that can use the inner shell before using the outer shell to bond o main-group element -- an element in the s-block ...
... o alkaline-earth metal -- one of the elements of Group 2 of the periodic table (beryllium, magnesium, calcium, strontium, barium, and radium) o transition element -- one of the metals that can use the inner shell before using the outer shell to bond o main-group element -- an element in the s-block ...
The Periodic Table
... Group 17: The Halogens Most reactive nonmetals, with Fluorine being the most reactive of all nonmetals Found in the rocks of Earth's crust and dissolved in sea water Exist as a gas at room temperature (F2 and Cl2), a liquid (Br2), and a solid (I2 and At) Charge is -1: 7 valence electrons ...
... Group 17: The Halogens Most reactive nonmetals, with Fluorine being the most reactive of all nonmetals Found in the rocks of Earth's crust and dissolved in sea water Exist as a gas at room temperature (F2 and Cl2), a liquid (Br2), and a solid (I2 and At) Charge is -1: 7 valence electrons ...
The Periodic Table Memorize which elements are gases and
... Mendeleev first arranged the elements in his periodic table according to atomic mass. Mosley derived the modern periodic law stating that elements are a function of their periodic number, thus the modern periodic table is arranged by atomic number and not atomic mass. A. Each element has a speci ...
... Mendeleev first arranged the elements in his periodic table according to atomic mass. Mosley derived the modern periodic law stating that elements are a function of their periodic number, thus the modern periodic table is arranged by atomic number and not atomic mass. A. Each element has a speci ...
Periodic Table
... For the third period (row) the atomic radius for each atom gets smaller across the table ...
... For the third period (row) the atomic radius for each atom gets smaller across the table ...
answers
... 13. What feature of an element determines its place on the periodic table? Elements on the periodic table are arranged by atomic number which describes how many protons are in the nucleus of the atom. 14. What are the 3 main types of elements? Of the 3 main groups which type is the most abundant? El ...
... 13. What feature of an element determines its place on the periodic table? Elements on the periodic table are arranged by atomic number which describes how many protons are in the nucleus of the atom. 14. What are the 3 main types of elements? Of the 3 main groups which type is the most abundant? El ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.