Elements, Compounds and Mixtures
... Use this slide to describe and explain Rutherford’s experiment. Rutherford designed the apparatus shown in figure (A) to study the scattering of alpha particles by gold. Students may have difficult with the concepts in this experiment because they lack the necessary physics background. To help stude ...
... Use this slide to describe and explain Rutherford’s experiment. Rutherford designed the apparatus shown in figure (A) to study the scattering of alpha particles by gold. Students may have difficult with the concepts in this experiment because they lack the necessary physics background. To help stude ...
Resonance Superfluidity in a Quantum Degenerate Fermi Gas
... behavior of the gap D 苷 Up 2 gfm . The critical temperature Tc can be related to the value of the gap at T 苷 0. For comparison, in superconductors the analogous gap is simply the binding energy of a fermion pair. ...
... behavior of the gap D 苷 Up 2 gfm . The critical temperature Tc can be related to the value of the gap at T 苷 0. For comparison, in superconductors the analogous gap is simply the binding energy of a fermion pair. ...
CHM2C1-B Physical Spectroscopy
... 3. Give the three quantum numbers that describe a 2s atomic orbital. p.14 1. Write down two possible sets of quantum numbers to describe an electron in a 3s atomic orbital. 2. If an electron has the quantum numbers n=2, l=1, ml =1 and ms=+ 21 which type of atomic orbital is it occupying? •First •Pre ...
... 3. Give the three quantum numbers that describe a 2s atomic orbital. p.14 1. Write down two possible sets of quantum numbers to describe an electron in a 3s atomic orbital. 2. If an electron has the quantum numbers n=2, l=1, ml =1 and ms=+ 21 which type of atomic orbital is it occupying? •First •Pre ...
Gas Chromatography 1 C i ( bil h ) Carrier gas (mobile phase
... conductivity thus any analyte will decreases it . In system with reference cell carrier without the sample goes through a reference cell. The difference between amount of heat loss from carrier alone and from carrier with a sample is the measured signal. The sensitivity is inversely proportional to ...
... conductivity thus any analyte will decreases it . In system with reference cell carrier without the sample goes through a reference cell. The difference between amount of heat loss from carrier alone and from carrier with a sample is the measured signal. The sensitivity is inversely proportional to ...
Ch 8 LAN 7th Intro Chem Gases Liquids and Solids
... (Permanent) Dipole-Dipole Forces • Dipole-dipole forces are much weaker than those in ‘true’ bonds, but the effects of large collections of dipole–dipole forces are immense – as can be seen by looking at the difference in boiling points between polar and nonpolar molecules ...
... (Permanent) Dipole-Dipole Forces • Dipole-dipole forces are much weaker than those in ‘true’ bonds, but the effects of large collections of dipole–dipole forces are immense – as can be seen by looking at the difference in boiling points between polar and nonpolar molecules ...
Chem1101 – Semester 1
... Heteronuclear molecules: are formed through the mixing of different atomic orbitals gives rise to asymmetric molecular orbitals The bond between two elements with different electronegativities will be polar. Th ...
... Heteronuclear molecules: are formed through the mixing of different atomic orbitals gives rise to asymmetric molecular orbitals The bond between two elements with different electronegativities will be polar. Th ...
solutions - Scarsdale Public Schools
... If a solution in contact with pure solvent across a semipermeable membrane is subjected to an external pressure, reverse osmosis occurs. The pressure will cause a net flow from the solution to the solvent. In reverse osmosis the semipermeable membrane acts as a "molecular filter" to remove solute pa ...
... If a solution in contact with pure solvent across a semipermeable membrane is subjected to an external pressure, reverse osmosis occurs. The pressure will cause a net flow from the solution to the solvent. In reverse osmosis the semipermeable membrane acts as a "molecular filter" to remove solute pa ...
Pressure Data - Moore Chemistry
... Use the kinetic molecular theory (KMT) to explain how certain physical properties of ideal gases differ from real gases Describe the conditions under which real gases deviate from "ideal" behavior Explain the five postulates of the kinetic molecular theory. Define pressure and standard pressure in t ...
... Use the kinetic molecular theory (KMT) to explain how certain physical properties of ideal gases differ from real gases Describe the conditions under which real gases deviate from "ideal" behavior Explain the five postulates of the kinetic molecular theory. Define pressure and standard pressure in t ...
Whole version
... Eden, or in medias res with the current events in Iraq, the calculation of chemical reactions can begin with an introduction to the quantum theory and quantum statistics, or in medias res with a definition of the chemical potential . The most simple way to do so is by using a direct measuring proced ...
... Eden, or in medias res with the current events in Iraq, the calculation of chemical reactions can begin with an introduction to the quantum theory and quantum statistics, or in medias res with a definition of the chemical potential . The most simple way to do so is by using a direct measuring proced ...
3, 4, 7, 8, 10, 11, 13, 16, 17, 21, 22 Problems
... The Thermodynamic Efficiency (e) is defined as: ...
... The Thermodynamic Efficiency (e) is defined as: ...
Optimal solutions to non-negative PARAFAC/multilinear NMF
... I actually mostly do nonnegativity on all modes because it makes physical sense. Well, I always start without them, and then I actually mostly do nonnegativity on all modes because it makes physical sense. Well, I always start without them, and then if I run into problems, I start imposing nonnegati ...
... I actually mostly do nonnegativity on all modes because it makes physical sense. Well, I always start without them, and then I actually mostly do nonnegativity on all modes because it makes physical sense. Well, I always start without them, and then if I run into problems, I start imposing nonnegati ...
Chemistry - MrMunnsClass
... person’s hand, the shape of the sample changes, but the composition of the material does not change. Slide 21 of 26 © Copyright Pearson Prentice Hall ...
... person’s hand, the shape of the sample changes, but the composition of the material does not change. Slide 21 of 26 © Copyright Pearson Prentice Hall ...
SOLID-STATE PHYSICS II 2007 O. Entin-Wohlman
... ∗ ∗ ∗ exercise: Prepare a similar table for the ions with partially filled f −shell (L = 3). Hund’s three rules determine the ground state(s) of the partially-filled ion. However, that ground state is still degenerate. Take for example, the case n = 2 in the Table. After applying Hund’s first and se ...
... ∗ ∗ ∗ exercise: Prepare a similar table for the ions with partially filled f −shell (L = 3). Hund’s three rules determine the ground state(s) of the partially-filled ion. However, that ground state is still degenerate. Take for example, the case n = 2 in the Table. After applying Hund’s first and se ...
Topic 1222 Equation of State: Real Gases: van der Waals and Other
... The properties of gases pose a formidable challenge for chemists who seek to understand their p-V-T properties. Chemists adopt an approach which starts by defining the properties of a (hypothetical) ideal gas (Topics 1220 and 2588). The fact that the properties of a given real gas are not ideal is u ...
... The properties of gases pose a formidable challenge for chemists who seek to understand their p-V-T properties. Chemists adopt an approach which starts by defining the properties of a (hypothetical) ideal gas (Topics 1220 and 2588). The fact that the properties of a given real gas are not ideal is u ...
Supplementary Notes - Word file (264 KB )
... olivine compressibility curve presented in the Supplementary Figure. For the MORB investigated here (CH98 DR11, of Jambon et al., 1986), we have fitted the shock wave compression data obtained by Rigden et al. (1988) from an average MORB of similar composition. In these fits, we have accounted for t ...
... olivine compressibility curve presented in the Supplementary Figure. For the MORB investigated here (CH98 DR11, of Jambon et al., 1986), we have fitted the shock wave compression data obtained by Rigden et al. (1988) from an average MORB of similar composition. In these fits, we have accounted for t ...
Examples of Colligative properties are
... occurs at a pressure of 1.0 atm is called the normal freezing point. The same entropy effects which cause the boiling point to be elevated in a solution cause the freezing point to be depressed. See the figure above for a visual representation of this Since at the freezing point, the solid and liqui ...
... occurs at a pressure of 1.0 atm is called the normal freezing point. The same entropy effects which cause the boiling point to be elevated in a solution cause the freezing point to be depressed. See the figure above for a visual representation of this Since at the freezing point, the solid and liqui ...
The nitrogen gas inflates the airbags which generally have a
... 1. Obtain 6 round pieces of an aluminum can by using a hole punch. Obtain three weigh boats, and put two of the aluminum pieces in each. (Each trial will use 2 pieces.) Weigh the aluminum pieces on an analytical balance by taring the empty weigh boat first. Record the exact mass of the aluminum piec ...
... 1. Obtain 6 round pieces of an aluminum can by using a hole punch. Obtain three weigh boats, and put two of the aluminum pieces in each. (Each trial will use 2 pieces.) Weigh the aluminum pieces on an analytical balance by taring the empty weigh boat first. Record the exact mass of the aluminum piec ...
Export To Word
... This is free, easy-to-read, and accessible book that explains the three states of matter. The book may be downloaded as slide Three Kinds of Matter: Solids, show in PowerPoint, Impress, or Flash format. The book can be Liquids, and Gases speech enabled and accessed using multiple interfaces, includi ...
... This is free, easy-to-read, and accessible book that explains the three states of matter. The book may be downloaded as slide Three Kinds of Matter: Solids, show in PowerPoint, Impress, or Flash format. The book can be Liquids, and Gases speech enabled and accessed using multiple interfaces, includi ...
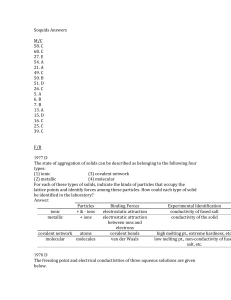
Soquids Answers M/C 58. C 68. C 27. E 54. A 21. A 49. C 50. B 51
... (b) (i) a solution made from a non-volatile solute has a higher boiling point than the pure solvent because the solution has a lower vapor pressure than the water (Raoult’s Law) . the temperature of the solution has be higher to produce enough vapor pressure to equal the atmospheric pressure (i.e., ...
... (b) (i) a solution made from a non-volatile solute has a higher boiling point than the pure solvent because the solution has a lower vapor pressure than the water (Raoult’s Law) . the temperature of the solution has be higher to produce enough vapor pressure to equal the atmospheric pressure (i.e., ...
CNT Sensors for Detecting Gases with Low Adsorption Energy by Ionization
... ionization sensors has been more prevailed due to easy thermionic emission of electrons from a cathode than the cold-cathode type by field emission, but the thermionic emission has some weak points; as pressure is very sensitive to temperature, the heat from filament must be blocked, and as chemical ...
... ionization sensors has been more prevailed due to easy thermionic emission of electrons from a cathode than the cold-cathode type by field emission, but the thermionic emission has some weak points; as pressure is very sensitive to temperature, the heat from filament must be blocked, and as chemical ...
Chemistry Entrance Material for Grade 10 to 11 Answer Key
... 9. Which one of the following is TRUE when a liquid is cooled? [-A-] A plot of temperature versus distance can be drawn. [-B-] A plot of temperature versus time can be drawn. [-C-] Energy is released at an increasing rate. [-D-] Energy is released at a constant rate. Comparing cooling curves of 2 sa ...
... 9. Which one of the following is TRUE when a liquid is cooled? [-A-] A plot of temperature versus distance can be drawn. [-B-] A plot of temperature versus time can be drawn. [-C-] Energy is released at an increasing rate. [-D-] Energy is released at a constant rate. Comparing cooling curves of 2 sa ...
CLASSIFICATION OF MATTER
... The elements are pure substances with a single type of atom; they cannot be broken down into simpler substances by chemical procedures. For example: carbon, iron, aluminium, etc. ...
... The elements are pure substances with a single type of atom; they cannot be broken down into simpler substances by chemical procedures. For example: carbon, iron, aluminium, etc. ...
Physical or Chemical Property?
... compacted in the tiny positively charged nucleus accounting for most of the mass of the atom • The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
... compacted in the tiny positively charged nucleus accounting for most of the mass of the atom • The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
Introduction to Soft Matter Physics
... Consider a material of thickness d sandwiched between two infinite plates. We apply a shear stress σs, which is the force that the upper plate is “pulled” with divided by its area (like a pressure). The plates move with a velocity v relative to each other with the material perfectly “attached” to th ...
... Consider a material of thickness d sandwiched between two infinite plates. We apply a shear stress σs, which is the force that the upper plate is “pulled” with divided by its area (like a pressure). The plates move with a velocity v relative to each other with the material perfectly “attached” to th ...
Lewis Structures Notes • Draw the dot diagram for
... Covalent bonds are between _____________________________________________. ...
... Covalent bonds are between _____________________________________________. ...