Band Theories
... If the atomic p orbitals lie higher in energy than the s orbitals, the the p band lies higher in energy than the s band and there may be a band gap – a range of energies to which no orbital corresponds. ...
... If the atomic p orbitals lie higher in energy than the s orbitals, the the p band lies higher in energy than the s band and there may be a band gap – a range of energies to which no orbital corresponds. ...
Exam 4 - Chemistry Courses
... compounds that are believed to be less harmful to the environment. What mass of this substance must evaporate (at constant T) in order to freeze 2 moles of water at 20 °C to ice at 0 °C? The heat of vaporization of CCl2F2 is 289 J/g, the heat of fusion of water is 334 J/g , and the specific heat of ...
... compounds that are believed to be less harmful to the environment. What mass of this substance must evaporate (at constant T) in order to freeze 2 moles of water at 20 °C to ice at 0 °C? The heat of vaporization of CCl2F2 is 289 J/g, the heat of fusion of water is 334 J/g , and the specific heat of ...
Sample pages 2 PDF
... The traditional thermometers consist of a glass bulb containing a liquid connected to a capillary tube several centimeters long. When in contact with a warmer body, the liquid expands; the higher the temperature, the higher it rises in the capillary. Mercury was in standard use as thermometer liquid ...
... The traditional thermometers consist of a glass bulb containing a liquid connected to a capillary tube several centimeters long. When in contact with a warmer body, the liquid expands; the higher the temperature, the higher it rises in the capillary. Mercury was in standard use as thermometer liquid ...
In situ-XAS and catalytic study of acrolein hydrogenation over silver
... species can be considered as a partly hydrogenated acrolein that after further hydrogen addition desorbs as propionaldehyde. We have shown above, that at low pressures propionaldehyde is preferentially formed. Parallel to this, at the silver surface flat lying propionaldehyde-like intermediate was o ...
... species can be considered as a partly hydrogenated acrolein that after further hydrogen addition desorbs as propionaldehyde. We have shown above, that at low pressures propionaldehyde is preferentially formed. Parallel to this, at the silver surface flat lying propionaldehyde-like intermediate was o ...
Colloidal Crystal: emergence of long range order from colloidal fluid
... and soft system. This size scale is especially interesting: it is close to biogical system so it is extremely informative for understanding life related phenomena, where emergence is origin of life itself; it is within visible light wavelength, so that it provides a model system for atomic system wi ...
... and soft system. This size scale is especially interesting: it is close to biogical system so it is extremely informative for understanding life related phenomena, where emergence is origin of life itself; it is within visible light wavelength, so that it provides a model system for atomic system wi ...
Plasma Physics Definitions
... elastic or inelastic. – Elastic collisions deplete very little of the electron’s energy and do not significantly influence the molecules because of the great mass difference between electrons and molecules: Mass of electron = 9.11 e-31 kg, Mass of Argon = 6.64e20 kg. – Inelastic collisions excite th ...
... elastic or inelastic. – Elastic collisions deplete very little of the electron’s energy and do not significantly influence the molecules because of the great mass difference between electrons and molecules: Mass of electron = 9.11 e-31 kg, Mass of Argon = 6.64e20 kg. – Inelastic collisions excite th ...
Document
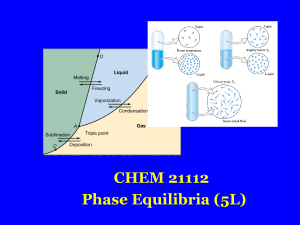
... Gibbs free energy. Phases, components and degree of freedom, the phase rule, phase diagrams; interpretation, lever rule. Liquid-liquid phase diagrams; phase separation, critical solution temperatures. Temperature-composition diagrams; distillation of mixtures, zeotropes and azeotropes. Liquid solid ...
... Gibbs free energy. Phases, components and degree of freedom, the phase rule, phase diagrams; interpretation, lever rule. Liquid-liquid phase diagrams; phase separation, critical solution temperatures. Temperature-composition diagrams; distillation of mixtures, zeotropes and azeotropes. Liquid solid ...
Document
... – Combination of concentrations that allow Q = K – Infinite number of possible equilibrium positions • Le Châtelier’s principle – System at equilibrium (Q = K) when upset by disturbance (Q ≠ K) will shift to offset stress • System said to “shift to right” when forward reaction is dominant (Q < K) • ...
... – Combination of concentrations that allow Q = K – Infinite number of possible equilibrium positions • Le Châtelier’s principle – System at equilibrium (Q = K) when upset by disturbance (Q ≠ K) will shift to offset stress • System said to “shift to right” when forward reaction is dominant (Q < K) • ...
fabrication of polymer form-silica nanocomposite thermal
... We have measured the phase diagram of several silicon alkoxide-CO2 binary systems and polymer-silicon alkoxide-CO2 ternary systems [1]. We have also explored preparation of polymer foam-silica nanocomposites by the above method in a batch reactor, and demonstrated that it is indeed to produce nanoco ...
... We have measured the phase diagram of several silicon alkoxide-CO2 binary systems and polymer-silicon alkoxide-CO2 ternary systems [1]. We have also explored preparation of polymer foam-silica nanocomposites by the above method in a batch reactor, and demonstrated that it is indeed to produce nanoco ...
AP CHEMISTRY COURSE SYLLABUS
... perform laboratory activities on any given day. THEMES: This course is centered on the 4 major themes, Structure of Matter, States of Matter, Chemical Reactions, and Descriptive Chemistry, listed in the AP Chemistry description. Within each of these themes, there will be topics that are extensions o ...
... perform laboratory activities on any given day. THEMES: This course is centered on the 4 major themes, Structure of Matter, States of Matter, Chemical Reactions, and Descriptive Chemistry, listed in the AP Chemistry description. Within each of these themes, there will be topics that are extensions o ...
Free sample of
... a. absorb low-energy x-rays b. remove high-energy x-rays c. restrict the useful beam to the body part imaged d. fabricate gonadal shields ANS: A Filtration is used to absorb low-energy x-rays. DIF: Moderate REF: page 10 OBJ: Relate history of the development of computed tomography. ...
... a. absorb low-energy x-rays b. remove high-energy x-rays c. restrict the useful beam to the body part imaged d. fabricate gonadal shields ANS: A Filtration is used to absorb low-energy x-rays. DIF: Moderate REF: page 10 OBJ: Relate history of the development of computed tomography. ...
Electron Shell Contributions to Gamma-ray Spectra of Positron Annihilation in Noble gases" J. Phys. B.: Atomic, Molecular and Optical Physics , 43 , 165207 (2010). Feng Wang, Lalitha Selvam, and C. M. Surko, Gleb F Gribakin, and C. M. Surko (PDF)
... Positron–electron annihilation spectra are very sensitive to the atomic electron shells where the bound electrons reside (i.e. to the principal quantum number n and the orbital angular quantum number l). Table 2 reports the bound electron contributions to the spectra of the noble gases. It is always ...
... Positron–electron annihilation spectra are very sensitive to the atomic electron shells where the bound electrons reside (i.e. to the principal quantum number n and the orbital angular quantum number l). Table 2 reports the bound electron contributions to the spectra of the noble gases. It is always ...
Pressure induced polymerization of acetylide anions in CaC2 and
... Fig. S1.† However, above 10–12 GPa, the hkl peaks with l s 0 are signicantly broadened, the C^C bond length decreases signicantly to unreasonable values and the nearest C/C intergroup distances increase correspondingly (Fig. 2b). This indicates that the CaC2-I model is not suitable for the data ab ...
... Fig. S1.† However, above 10–12 GPa, the hkl peaks with l s 0 are signicantly broadened, the C^C bond length decreases signicantly to unreasonable values and the nearest C/C intergroup distances increase correspondingly (Fig. 2b). This indicates that the CaC2-I model is not suitable for the data ab ...
The Formation of Solvated Electrons in the Photochemistry of the
... The Photochemistry of Phenol in Aqueous Solutions.-It should be added a t this point that solvated electrons are not formed, or at most are formed with very low quantum vields, on irradiation of neutral or acid aqueous solutions of phenol a t 2288 A. This was confirmed by two experiments in which 0. ...
... The Photochemistry of Phenol in Aqueous Solutions.-It should be added a t this point that solvated electrons are not formed, or at most are formed with very low quantum vields, on irradiation of neutral or acid aqueous solutions of phenol a t 2288 A. This was confirmed by two experiments in which 0. ...
Concentration of solutions
... the solubility of a gas in a liquid is Directly proportional to the partial pressure of that gas on the surface of the liquid. S1 = S2 P1 P2 In carbonated beverages forcing it into solution at pressure of 5-10 atm increases CO2 solubility. The containers are then sealed. When opened, the CO2 gas esc ...
... the solubility of a gas in a liquid is Directly proportional to the partial pressure of that gas on the surface of the liquid. S1 = S2 P1 P2 In carbonated beverages forcing it into solution at pressure of 5-10 atm increases CO2 solubility. The containers are then sealed. When opened, the CO2 gas esc ...
Metamorphism / Metamorphic Rocks Metamorphism
... Metamorphism: occurs when rocks are subjected to heat, pressure, and/or other environmental conditions - The rock remains a solid during this time period - Why Should You Study Metamorphic Rocks? - Exposed metamorphic rocks make up large parts of continents - Certain minerals in metamorphic rocks gi ...
... Metamorphism: occurs when rocks are subjected to heat, pressure, and/or other environmental conditions - The rock remains a solid during this time period - Why Should You Study Metamorphic Rocks? - Exposed metamorphic rocks make up large parts of continents - Certain minerals in metamorphic rocks gi ...
Chem101 - Lecture 1 - chem.uwec.edu
... white solid compares with the size of the molecules of phosphorus? Explain. Classify the molecules of the collected white solid using the terms homoatomic and ...
... white solid compares with the size of the molecules of phosphorus? Explain. Classify the molecules of the collected white solid using the terms homoatomic and ...
syllabus - WordPress.com
... Explain the term associated colloids (Micelles). There are some substances which at low concentrations behave as normal strong electrolytes, but at higher concentrations exhibit colloidal behaviour due to the formation of aggregates. The aggregated particles thus formed are called micelles. These ar ...
... Explain the term associated colloids (Micelles). There are some substances which at low concentrations behave as normal strong electrolytes, but at higher concentrations exhibit colloidal behaviour due to the formation of aggregates. The aggregated particles thus formed are called micelles. These ar ...
Liquid-gas transition of neon in quasi-one
... gas-liquid transition critical temperature in the case of Ne, using a 3D modified anisotropic Ising model to get T c . 31 A comparison between the number obtained in that approximation (T c ⫽63.8 K) and the one in the present work shows that in the neon case that Ising model is completely inadequate ...
... gas-liquid transition critical temperature in the case of Ne, using a 3D modified anisotropic Ising model to get T c . 31 A comparison between the number obtained in that approximation (T c ⫽63.8 K) and the one in the present work shows that in the neon case that Ising model is completely inadequate ...
Balancing Chemical Equations
... Atomic and molecular weight The atomic weight is the numerical value tabulated for the mass of each atom in the periodic table in atomic units. The use of the word “weight” is not precise here since weight in physics represents a force (w = mg). However, the name atomic weight is so ingrained that ...
... Atomic and molecular weight The atomic weight is the numerical value tabulated for the mass of each atom in the periodic table in atomic units. The use of the word “weight” is not precise here since weight in physics represents a force (w = mg). However, the name atomic weight is so ingrained that ...
Sample Paper
... is formed via electrolysis in the nucleation core and enters the measurement channel as it grows (Fig. 1a), forming capillaries of fluid in the channel corners (Fig. 2). Once a sufficient size is reached, electrolysis current is terminated and the B detaches from the nucleation core. The electrical ...
... is formed via electrolysis in the nucleation core and enters the measurement channel as it grows (Fig. 1a), forming capillaries of fluid in the channel corners (Fig. 2). Once a sufficient size is reached, electrolysis current is terminated and the B detaches from the nucleation core. The electrical ...
HEFAT2014 10 International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics
... means of heat conductivity and the energy flux density is set by the Fourier's law, T , where is the material conductivity, T is the temperature [1]. Nevertheless, the electrical currents of electrons and holes can be presented in bipolar semiconductors when the total electrical current is abse ...
... means of heat conductivity and the energy flux density is set by the Fourier's law, T , where is the material conductivity, T is the temperature [1]. Nevertheless, the electrical currents of electrons and holes can be presented in bipolar semiconductors when the total electrical current is abse ...
The Ideal Gas Equation
... 1) A sample of gas assumes both the shape and volume of the container. 2) Gases are compressible. 3) The densities of gases are much smaller than those of liquids and solids and are highly variable depending on temperature and pressure. 4) Gases form homogeneous mixtures (solutions) with one another ...
... 1) A sample of gas assumes both the shape and volume of the container. 2) Gases are compressible. 3) The densities of gases are much smaller than those of liquids and solids and are highly variable depending on temperature and pressure. 4) Gases form homogeneous mixtures (solutions) with one another ...
AP Lab - MW of Volatile Liquid - North Allegheny School District
... If a substance behaves as an ideal gas, we can easily calculate its molar mass if we measure the mass m of a volume V of the pure gas at known temperature T and pressure P. The ideal gas law is PV = nRT, where n = moles of gas. But n = m/Mw where Mw equals molar mass. Therefore, Mw = mRT/PV. This me ...
... If a substance behaves as an ideal gas, we can easily calculate its molar mass if we measure the mass m of a volume V of the pure gas at known temperature T and pressure P. The ideal gas law is PV = nRT, where n = moles of gas. But n = m/Mw where Mw equals molar mass. Therefore, Mw = mRT/PV. This me ...