10.1 The Mole: A Measure
... • For elements, this is the atomic mass given in the periodic table, expressed in grams. • Also called molecular weight or molecular mass(in case of molecules which is the sum of atomic masses of all the atoms they are composed of) Element Carbon Hydrogen Chlorine ...
... • For elements, this is the atomic mass given in the periodic table, expressed in grams. • Also called molecular weight or molecular mass(in case of molecules which is the sum of atomic masses of all the atoms they are composed of) Element Carbon Hydrogen Chlorine ...
Suggested Student Schedule (progress chart – to be
... Learning Outcomes: Be able to describe the various forms of energy and understand concept of conservation of energy ...
... Learning Outcomes: Be able to describe the various forms of energy and understand concept of conservation of energy ...
Chem 310 Lectures by: Dr. Muhammad D. Bala Office: Block H, 3
... Degenerate orbitals are filled according to Hund's rules: • One electron is added to each of the degenerate orbitals in a subshell before a second electron is added to any orbital in the subshell Î lowest energy subshell filled in first. • Electrons are added to a subshell with the same value of the ...
... Degenerate orbitals are filled according to Hund's rules: • One electron is added to each of the degenerate orbitals in a subshell before a second electron is added to any orbital in the subshell Î lowest energy subshell filled in first. • Electrons are added to a subshell with the same value of the ...
Solutions
... When a solution contains the maximum amount of solute that will dissolve at a specific temperature it is saturated. In this case the amount dissolved will be directly on the line of solubility. Solution equilibrium. When a solution contains more than the maximum amount of solute that will dissolve a ...
... When a solution contains the maximum amount of solute that will dissolve at a specific temperature it is saturated. In this case the amount dissolved will be directly on the line of solubility. Solution equilibrium. When a solution contains more than the maximum amount of solute that will dissolve a ...
FeCo magnetic nanoneedles obtained by Co-coating
... In order to obtain magnetite particles with Co content up to 30%, sample 10-CoM was coated with an additional 10 and 20% of Co, using the same procedure as that used with haematite and then subjected again to the first reduction step (see figure 1). In this way, single-phase magnetite particles with ...
... In order to obtain magnetite particles with Co content up to 30%, sample 10-CoM was coated with an additional 10 and 20% of Co, using the same procedure as that used with haematite and then subjected again to the first reduction step (see figure 1). In this way, single-phase magnetite particles with ...
Nugget
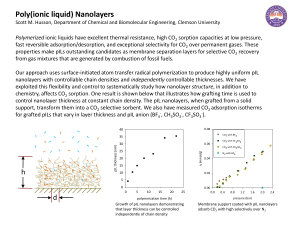
... fast reversible adsorption/desorption, and exceptional selectivity for CO2 over permanent gases. These properties make pILs outstanding candidates as membrane separation layers for selective CO2 recovery from gas mixtures that are generated by combustion of fossil fuels. ...
... fast reversible adsorption/desorption, and exceptional selectivity for CO2 over permanent gases. These properties make pILs outstanding candidates as membrane separation layers for selective CO2 recovery from gas mixtures that are generated by combustion of fossil fuels. ...
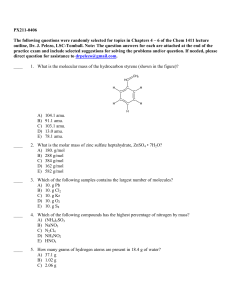
PX211-0406 The following questions were randomly selected for
... 29. Which of the following statements is least likely to be true of a sample of nitrogen gas at STP? A) Collisions between the gaseous molecules are elastic. B) The intermolecular forces between nitrogen molecules are not negligible. C) Molecules of gaseous nitrogen are in constant random motion. D) ...
... 29. Which of the following statements is least likely to be true of a sample of nitrogen gas at STP? A) Collisions between the gaseous molecules are elastic. B) The intermolecular forces between nitrogen molecules are not negligible. C) Molecules of gaseous nitrogen are in constant random motion. D) ...
Nordonia Hills City Schools Honors Chemistry Course of Study
... Determine the number of protons, electrons and neutrons; write nuclide symbols Describe properties, names, and location of subatomic particles. Compare and contrast contributors to early atomic theory: Greeks, Dalton, Thomson, Rutherford, Chadwick, and Bohr) Describe concepts involved in Dalton's po ...
... Determine the number of protons, electrons and neutrons; write nuclide symbols Describe properties, names, and location of subatomic particles. Compare and contrast contributors to early atomic theory: Greeks, Dalton, Thomson, Rutherford, Chadwick, and Bohr) Describe concepts involved in Dalton's po ...
Chm 118
... Imagine a system with energy spacing of two units, comprised of 20/2/0 particles for a total energy of 4 units. Hold the energy constant, but increase the size of the container such that the spacing between the levels becomes one unit. What configuration is roughly consistent with 4 units of energy ...
... Imagine a system with energy spacing of two units, comprised of 20/2/0 particles for a total energy of 4 units. Hold the energy constant, but increase the size of the container such that the spacing between the levels becomes one unit. What configuration is roughly consistent with 4 units of energy ...
Growing Negative Pressure in Dissolved Solutes: Raman - HAL-Insu
... Negative pressure is still nowadays a paradoxical-sounding concept, because the notion collides between gases, for which the zero pressure is an absolute limit (pressure and matter density are strictly proportional at low density), and condensed matter, the internal cohesion of which allows them to ...
... Negative pressure is still nowadays a paradoxical-sounding concept, because the notion collides between gases, for which the zero pressure is an absolute limit (pressure and matter density are strictly proportional at low density), and condensed matter, the internal cohesion of which allows them to ...
Synthesis and Characterization of Large Colloidal Silver Particles
... Au, Al, Ni, Cu) have been investigated theoretically as possible candidates for metallodielectric photonic crystals.10-14 Because of its low bulk absorption, silver (Ag) is the most suitable metal to create a CPBG in the visible. Recent calculations of Moroz have shown that a CPBG can even be opened ...
... Au, Al, Ni, Cu) have been investigated theoretically as possible candidates for metallodielectric photonic crystals.10-14 Because of its low bulk absorption, silver (Ag) is the most suitable metal to create a CPBG in the visible. Recent calculations of Moroz have shown that a CPBG can even be opened ...
On the turbulence structure in inert and reacting
... number based on the local density and viscosity, the values in the centre of the reacting mixing layers are reduced by a factor of about 6. Figure 1 shows the spectra of the turbulent kinetic energy at the beginning of the self-similar state. The energy cascades are well established in all cases. At ...
... number based on the local density and viscosity, the values in the centre of the reacting mixing layers are reduced by a factor of about 6. Figure 1 shows the spectra of the turbulent kinetic energy at the beginning of the self-similar state. The energy cascades are well established in all cases. At ...
Chemistry Content Review Notes
... (VDOE) Curriculum Framework, Enhanced Scope and Sequence, and Released Test items. In addition to VDOE information, Glencoe Textbook Series and resources have been used. Finally, information from various websites is included. The websites are listed with the information as it appears in the document ...
... (VDOE) Curriculum Framework, Enhanced Scope and Sequence, and Released Test items. In addition to VDOE information, Glencoe Textbook Series and resources have been used. Finally, information from various websites is included. The websites are listed with the information as it appears in the document ...
• • • • • • • • • • • • • • • • • • • • • • • • • •
... to examine the fundamental concepts of nuclear and organic chemistry. The course provides many opportunities for the student to apply these concepts to real-world situations. 1. Solids, Liquids, and Gases 1. The Nature of Gases Describe the assumptions of the kinetic theory as it applies to gases. ...
... to examine the fundamental concepts of nuclear and organic chemistry. The course provides many opportunities for the student to apply these concepts to real-world situations. 1. Solids, Liquids, and Gases 1. The Nature of Gases Describe the assumptions of the kinetic theory as it applies to gases. ...
Chapter 9 slides
... P1V1 = P2V2 If the pressure of a 2.0-L sample of gas is decreased from 1.2 atm to 0.25 atm at constant temperature, what is the new volume? ...
... P1V1 = P2V2 If the pressure of a 2.0-L sample of gas is decreased from 1.2 atm to 0.25 atm at constant temperature, what is the new volume? ...
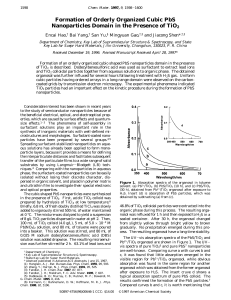
Formation of Orderly Organized Cubic PbS Nanoparticles Domain in
... the beneficial electrical, optical, and electrooptical properties, which are caused by surface effects and quantumsize effects.1-3 The phenomena of self-assembly in surfactant solutions play an important role in the synthesis of inorganic materials with well-defined microstructures and morphologies. ...
... the beneficial electrical, optical, and electrooptical properties, which are caused by surface effects and quantumsize effects.1-3 The phenomena of self-assembly in surfactant solutions play an important role in the synthesis of inorganic materials with well-defined microstructures and morphologies. ...
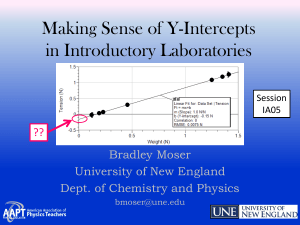
Making Sense of Y-Intercepts in Introductory Laboratories
... either for imparting or extracting information.” • “Among the many skills that can be developed in the study of physics, the ability to draw and interpret graphs is perhaps one of the most important.” McDermott : American Journal of Physics 55, 503 (1987) ...
... either for imparting or extracting information.” • “Among the many skills that can be developed in the study of physics, the ability to draw and interpret graphs is perhaps one of the most important.” McDermott : American Journal of Physics 55, 503 (1987) ...
IV. The Transmission Electron Microscope
... will support pressure ratios of approximately 10:1 or greater. Typically there are three jet assemblies of diminishing sizes, with the largest at the bottom. The pressure on the low-pressure side is typically around 10-4 Pa or so, while the maximum pressure on the high pressure side is typically on ...
... will support pressure ratios of approximately 10:1 or greater. Typically there are three jet assemblies of diminishing sizes, with the largest at the bottom. The pressure on the low-pressure side is typically around 10-4 Pa or so, while the maximum pressure on the high pressure side is typically on ...
co2 removal from natural gas by hydrate formation
... the system, which is confirmed by the direct observation through the sapphire windows (snapshot B). When the heat released by hydrate crystallization is offset by the cell cooling, the temperature of the system decreases to point C, where a second hydrate crystallization takes place. These two steps ...
... the system, which is confirmed by the direct observation through the sapphire windows (snapshot B). When the heat released by hydrate crystallization is offset by the cell cooling, the temperature of the system decreases to point C, where a second hydrate crystallization takes place. These two steps ...
Solutions
... When a solution contains the maximum amount of solute that will dissolve at a specific temperature it is_________. In this case the amount dissolved will be _________the line of solubility. Solution____________. When a solution contains more than the maximum amount of solute that will dissolve at a ...
... When a solution contains the maximum amount of solute that will dissolve at a specific temperature it is_________. In this case the amount dissolved will be _________the line of solubility. Solution____________. When a solution contains more than the maximum amount of solute that will dissolve at a ...
Key concepts from last class • The internal energy, U, is the total
... • For a monoatomic ideal gas, the only contribution is the translational kinetic energy, ...
... • For a monoatomic ideal gas, the only contribution is the translational kinetic energy, ...
types of solutions
... · Physical properties of solutions that depend on the concentration, but not the type of solute particles are called colligative properties. · Examples of such properties are: Lowering of freezing point Elevation of boiling point Lowering of osmotic pressure ...
... · Physical properties of solutions that depend on the concentration, but not the type of solute particles are called colligative properties. · Examples of such properties are: Lowering of freezing point Elevation of boiling point Lowering of osmotic pressure ...
Phase Transformations Some Definitions Some Definitions, 2
... Phase diagrams are extremely useful for systems with multiple components, and serve to describe physical and chemical equilibria over a range of different compositions, as well as points where substances are mutually miscible, or even when a system has to be brought to a specific set of conditions f ...
... Phase diagrams are extremely useful for systems with multiple components, and serve to describe physical and chemical equilibria over a range of different compositions, as well as points where substances are mutually miscible, or even when a system has to be brought to a specific set of conditions f ...
Activity (chemistry) - Chemical Engineering
... condensed phases (solid or liquids) is normally taken as unity. Activity depends on temperature, pressure and composition of the mixture, among other things. For gases, the effective partial pressure is usually referred to as fugacity. The difference between activity and other measures of compositio ...
... condensed phases (solid or liquids) is normally taken as unity. Activity depends on temperature, pressure and composition of the mixture, among other things. For gases, the effective partial pressure is usually referred to as fugacity. The difference between activity and other measures of compositio ...