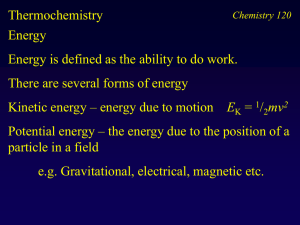
Thermochemistry Energy Energy is defined as the ability to do work
... The temperature and pressure The molecular structure of the system As 1 mole = 6.02 x 1023 particles, defining the state of a system uniquely is experimentally impossible in an absolute sense. ...
... The temperature and pressure The molecular structure of the system As 1 mole = 6.02 x 1023 particles, defining the state of a system uniquely is experimentally impossible in an absolute sense. ...
Thermal and Statistical Physics
... The ideas and methods developed in this course find very broad application, not only within physics, but in biology, geology, chemistry, astronomy, engineering, computer science/artificial intelligence/information technology, finance, philosophy, etc. Indeed one of the central reasons why a physics deg ...
... The ideas and methods developed in this course find very broad application, not only within physics, but in biology, geology, chemistry, astronomy, engineering, computer science/artificial intelligence/information technology, finance, philosophy, etc. Indeed one of the central reasons why a physics deg ...
Chemistry 120
... Most reactions take place at constant pressure and therefore we define a new function, which is a state function in the same way that U is a state function ...
... Most reactions take place at constant pressure and therefore we define a new function, which is a state function in the same way that U is a state function ...
chromapp
... • sufficient energy is given to form ions of 1+ charge ACCELERATION • ions are charged so can be ACCELERATED by an electric field DEFLECTION • charged particles will be DEFLECTED by a magnetic or electric field DETECTION • by electric or photographic methods For more information, consult the notes o ...
... • sufficient energy is given to form ions of 1+ charge ACCELERATION • ions are charged so can be ACCELERATED by an electric field DEFLECTION • charged particles will be DEFLECTED by a magnetic or electric field DETECTION • by electric or photographic methods For more information, consult the notes o ...
No Slide Title
... • sufficient energy is given to form ions of 1+ charge ACCELERATION • ions are charged so can be ACCELERATED by an electric field DEFLECTION • charged particles will be DEFLECTED by a magnetic or electric field DETECTION • by electric or photographic methods For more information, consult the notes o ...
... • sufficient energy is given to form ions of 1+ charge ACCELERATION • ions are charged so can be ACCELERATED by an electric field DEFLECTION • charged particles will be DEFLECTED by a magnetic or electric field DETECTION • by electric or photographic methods For more information, consult the notes o ...
“Hidden” Momentum in a Sound Wave
... In certain electromechanical systems where mechanical momentum of order 1/c2 is present in association with electromagnetic momentum, the term “hidden” momentum has come into use [2, 6, 7, 8, 9, 11, 10, 12, 13, 14, 15, 16]. According to the general definition (1), the present system as a whole does n ...
... In certain electromechanical systems where mechanical momentum of order 1/c2 is present in association with electromagnetic momentum, the term “hidden” momentum has come into use [2, 6, 7, 8, 9, 11, 10, 12, 13, 14, 15, 16]. According to the general definition (1), the present system as a whole does n ...
1.2 Calculations
... If titrating a mixture to work out the concentration of an active ingredient it is necessary to consider if the mixture contains other substances that have acid base properties. If they don’t have acid base properties we can titrate with ...
... If titrating a mixture to work out the concentration of an active ingredient it is necessary to consider if the mixture contains other substances that have acid base properties. If they don’t have acid base properties we can titrate with ...
Low-Energy (20 eV) and High-Energy (1000 eV) Electron
... coverage of CH3OH. One monolayer (1 ML) is defined as the coverage achieved by the maximum exposure of the adsorbate that does not yield a multilayer peak. Film thickness (20–100 ML) was sufficient to rule out any contribution from Mo surface ...
... coverage of CH3OH. One monolayer (1 ML) is defined as the coverage achieved by the maximum exposure of the adsorbate that does not yield a multilayer peak. Film thickness (20–100 ML) was sufficient to rule out any contribution from Mo surface ...
Modeling the extraction of sputtered metal from Linköping University Post Print
... discharge in a mode that creates a favorable plasma environment for size control and efficient growth of nanoparticles in the expansion volume below the hollow cathode. The desirable environment is described in a patent [11]. There it is shown that a high degree of ionization is beneficial for the g ...
... discharge in a mode that creates a favorable plasma environment for size control and efficient growth of nanoparticles in the expansion volume below the hollow cathode. The desirable environment is described in a patent [11]. There it is shown that a high degree of ionization is beneficial for the g ...
Induction plasma spheroidization of nanometric glass powder for use in cementations materials
... WG into spheroidized glass powder (SGP) that could be used as a cementitious material. Changing the WG feed rate and pressure induce variations on the particle size distribution and chemical composition. Using Field Emission Gun Scanning Electron Microscopy (FEGSEM), Brunauer, Emmet and Teller (BET) ...
... WG into spheroidized glass powder (SGP) that could be used as a cementitious material. Changing the WG feed rate and pressure induce variations on the particle size distribution and chemical composition. Using Field Emission Gun Scanning Electron Microscopy (FEGSEM), Brunauer, Emmet and Teller (BET) ...
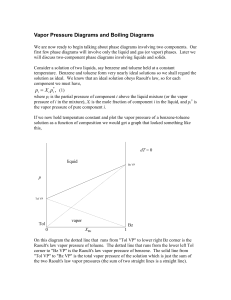
Vapor Pressure Diagrams and Boiling Diagrams
... As we heat the liquid it will begin to boil when the temperature reaches the temperature of point "a." The first vapor to come off has the composition shown at point "b." Capture the vapor, condense it, and heat it up. The new liquid will boil at point "c" giving a vapor with composition at point "d ...
... As we heat the liquid it will begin to boil when the temperature reaches the temperature of point "a." The first vapor to come off has the composition shown at point "b." Capture the vapor, condense it, and heat it up. The new liquid will boil at point "c" giving a vapor with composition at point "d ...
Ch. 14 Study Guide
... 20. If an equilibrium cannot be established, then the solution is unsaturated. 21. Supersaturated solutions hold more solute than is theoretically possible. 22. Generally, higher temperatures result in higher solubility of solids. 23. Henry’s Law states that if we push a gas hard enough (with extern ...
... 20. If an equilibrium cannot be established, then the solution is unsaturated. 21. Supersaturated solutions hold more solute than is theoretically possible. 22. Generally, higher temperatures result in higher solubility of solids. 23. Henry’s Law states that if we push a gas hard enough (with extern ...
Ignition Processes in Hydrogen
... Ignition processes in the hydrogen-oxygen system were simulated by solving the corresponding conservation equations (i.e., conservation of mass, energy, momentum, and species mass) for one-dimensional geometries using a detailed reaction mechanism and a mUltispecies transport model. An additional so ...
... Ignition processes in the hydrogen-oxygen system were simulated by solving the corresponding conservation equations (i.e., conservation of mass, energy, momentum, and species mass) for one-dimensional geometries using a detailed reaction mechanism and a mUltispecies transport model. An additional so ...
Mole-Mass Conversions
... Unlike mole-particle conversions where the conversion factor is always 1mole = _______________ particles, each mole-mass conversion factor is _________________ to the substance involved. Convert 2.3 moles of sodium (Na) to grams of Na ...
... Unlike mole-particle conversions where the conversion factor is always 1mole = _______________ particles, each mole-mass conversion factor is _________________ to the substance involved. Convert 2.3 moles of sodium (Na) to grams of Na ...
KINETICS (chap 12)
... IMF's - know the def and be able to identify the predominant IMF in a molecule ionic; network covalent; H-bond; dipole-dipole; LD, London Dispersion; IMF's and relation to boiling point, melting point and vapor pressure. Types of phase changes Be able to label and read a heating/cooling curve and a ...
... IMF's - know the def and be able to identify the predominant IMF in a molecule ionic; network covalent; H-bond; dipole-dipole; LD, London Dispersion; IMF's and relation to boiling point, melting point and vapor pressure. Types of phase changes Be able to label and read a heating/cooling curve and a ...
1 SOLUTIONS
... · The Solubility of gases decreases with increasing temperature (the colder the water, the better the gases dissolve). Examples are: Ø soda water keeps its carbonation (CO2 gas) better at low temperatures (at room temperature they go “flat” faster) Ø cold oceans have a higher concentration ...
... · The Solubility of gases decreases with increasing temperature (the colder the water, the better the gases dissolve). Examples are: Ø soda water keeps its carbonation (CO2 gas) better at low temperatures (at room temperature they go “flat” faster) Ø cold oceans have a higher concentration ...
Real gas CFD simulations of hydrogen/oxygen supercritical
... A modi¦ed SoaveRedlichKwong EOS and consistent real gas thermodynamics were also used by Ribert et al. [12] who studied counter§ow di¨usion §ames in the physical space over the entire regime of thermodynamic states. Signi¦cant real gas e¨ects due to steep property variations were found in the tran ...
... A modi¦ed SoaveRedlichKwong EOS and consistent real gas thermodynamics were also used by Ribert et al. [12] who studied counter§ow di¨usion §ames in the physical space over the entire regime of thermodynamic states. Signi¦cant real gas e¨ects due to steep property variations were found in the tran ...
Chemistry - Chillicothe City Schools
... reactant atoms and energy is released when an interaction or bond is formed between the atoms in the products. Essential Question #1: What are the different types of energy and energy transfer? Big Idea #3: All reactions are reversible to a degree and reactions tend toward a state of equilibrium ...
... reactant atoms and energy is released when an interaction or bond is formed between the atoms in the products. Essential Question #1: What are the different types of energy and energy transfer? Big Idea #3: All reactions are reversible to a degree and reactions tend toward a state of equilibrium ...
Solutions
... the vapor pressure of pure CS2 at 25°C is 358mmHg. Assume that the vapor pressure exerted by Naphthalene at 25°C is negligible. What is the vapor pressure of the solution at this temperature? ...
... the vapor pressure of pure CS2 at 25°C is 358mmHg. Assume that the vapor pressure exerted by Naphthalene at 25°C is negligible. What is the vapor pressure of the solution at this temperature? ...
Temperature and solid properties effects on gas–liquid mass transfer
... (PVC) beads and air/water/expandable polystyrene (EPS) beads were used. For each system, volumetric liquid side mass transfer coefficient, kL a, was determined under different temperatures (20–35 ◦ C), superficial gas velocities (up to 7.2 mm/s), solids sizes (210, 549 and 591 m) and concentration (u ...
... (PVC) beads and air/water/expandable polystyrene (EPS) beads were used. For each system, volumetric liquid side mass transfer coefficient, kL a, was determined under different temperatures (20–35 ◦ C), superficial gas velocities (up to 7.2 mm/s), solids sizes (210, 549 and 591 m) and concentration (u ...
Lecture Notes in Physical Chemistry Semester 2: Kinetics and
... (Notice that the exponent on the normalization factor is now 3/2.) If you think of the function d N v x v y v z /N as living in a three-dimensional “velocity space” whose axes are v x , v y , and v z , then the dv x dv y dv z part of Eq. (1.31) describes the volume of a small rectangular box, which ...
... (Notice that the exponent on the normalization factor is now 3/2.) If you think of the function d N v x v y v z /N as living in a three-dimensional “velocity space” whose axes are v x , v y , and v z , then the dv x dv y dv z part of Eq. (1.31) describes the volume of a small rectangular box, which ...
Tunneling through a Barrier
... • Tunnelling is very important for electrons and muons, and moderately important for protons; for heavier particles it is less important. • A number of effects in chemistry (e.g., the isotope-dependence of some reaction rates) depend on the ability of the proton to tunnel more readily than the deute ...
... • Tunnelling is very important for electrons and muons, and moderately important for protons; for heavier particles it is less important. • A number of effects in chemistry (e.g., the isotope-dependence of some reaction rates) depend on the ability of the proton to tunnel more readily than the deute ...
Investigation of Atmospheric Pressure Plasma Source for CO Dissociation
... with a higher flow rate of Ar can produce more electrons leading to higher conversions of CO2 to CO. As discussed in [12], an energy efficiency greater than 50% must be achieved for this technology to be of practical use when a fossil fuel such as natural gas provides the energy source. At a level o ...
... with a higher flow rate of Ar can produce more electrons leading to higher conversions of CO2 to CO. As discussed in [12], an energy efficiency greater than 50% must be achieved for this technology to be of practical use when a fossil fuel such as natural gas provides the energy source. At a level o ...
CHAPTER 11
... Figure 1.3, was introduced by Evangelista Torricelli during the early 1600s. Torricelli wondered why water pumps could raise water to a maximum height of only about 34 feet. He thought that the height must depend somehow on the weight of water compared with the weight of air. He reasoned that liquid ...
... Figure 1.3, was introduced by Evangelista Torricelli during the early 1600s. Torricelli wondered why water pumps could raise water to a maximum height of only about 34 feet. He thought that the height must depend somehow on the weight of water compared with the weight of air. He reasoned that liquid ...