ExamView - Untitled.tst
... ____ 15. What is the mass of 2.5 mol of Ca, which has a molar mass of 40 g/mol? a. 2.5 g Ca c. 40.0 g Ca b. 20.0 g Ca d. 100.0 g Ca ____ 16. An electron jumps to a new energy level when a. the atom becomes charged. b. the atom becomes unstable. c. the electron’s location is pinpointed. d. the atom g ...
... ____ 15. What is the mass of 2.5 mol of Ca, which has a molar mass of 40 g/mol? a. 2.5 g Ca c. 40.0 g Ca b. 20.0 g Ca d. 100.0 g Ca ____ 16. An electron jumps to a new energy level when a. the atom becomes charged. b. the atom becomes unstable. c. the electron’s location is pinpointed. d. the atom g ...
class notes packet - Social Circle City Schools
... Chromatography is a method for analyzing complex _______________ such as _____ by separating them into the chemicals fro which they are made. Attraction to a medium will take light particles up the medium as heavier particles stay low on the medium When can this be used? ...
... Chromatography is a method for analyzing complex _______________ such as _____ by separating them into the chemicals fro which they are made. Attraction to a medium will take light particles up the medium as heavier particles stay low on the medium When can this be used? ...
States of Matter
... stronger forces Polarizability of electron cloud – larger atoms’ electron clouds are more polarizable due to ...
... stronger forces Polarizability of electron cloud – larger atoms’ electron clouds are more polarizable due to ...
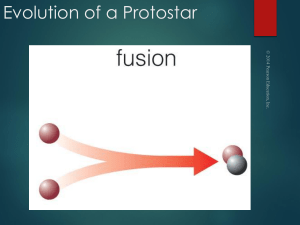
Evolution of a Protostar
... The contraction of a cloud fragment slows when thermal pressure builds up because infrared and radio photons can no longer escape. ...
... The contraction of a cloud fragment slows when thermal pressure builds up because infrared and radio photons can no longer escape. ...
Quantum Numbers and Atomic Structure Honors
... Quantum Numbers and Atomic Structure Honors Chemistry Please do not write on this exam 1. In an atom of argon-40, the number of protons A) B) C) D) ...
... Quantum Numbers and Atomic Structure Honors Chemistry Please do not write on this exam 1. In an atom of argon-40, the number of protons A) B) C) D) ...
Stars - Red, Blue, Old, New pt.4
... of the outer part-that’s normal matter, so the white dwarf does radiate according to its surface temp • 70,000-5000 K ...
... of the outer part-that’s normal matter, so the white dwarf does radiate according to its surface temp • 70,000-5000 K ...
Stages 12 to 14
... The carbon rich core continues to contract and heat up. Carbon fusion requires a temperature of 500 to 600 million K. The core will contract until electron degeneracy pressure once again takes over, and contraction ends If the star is similar to the sun, the mass is too small, the ignition temperatu ...
... The carbon rich core continues to contract and heat up. Carbon fusion requires a temperature of 500 to 600 million K. The core will contract until electron degeneracy pressure once again takes over, and contraction ends If the star is similar to the sun, the mass is too small, the ignition temperatu ...
Summer Assignment 2015
... 5. Give the complete chemical symbol for the atom that contains 82 protons, 82 electrons, and 126 neutrons. 6. Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, the ...
... 5. Give the complete chemical symbol for the atom that contains 82 protons, 82 electrons, and 126 neutrons. 6. Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, the ...
AST 301 Introduction to Astronomy - University of Texas Astronomy
... Normal gas and degenerate gas Pressure is the force a gas exerts on its surroundings. It is caused by the motion of the atoms. Or in a star, it is mostly the free electrons that cause pressure, since it is too hot for atoms to hold onto their electrons. In a normal gas, the electrons’ motion is cau ...
... Normal gas and degenerate gas Pressure is the force a gas exerts on its surroundings. It is caused by the motion of the atoms. Or in a star, it is mostly the free electrons that cause pressure, since it is too hot for atoms to hold onto their electrons. In a normal gas, the electrons’ motion is cau ...
electron degeneracy pressure and white dwarfs
... Most stable stars are stable because their weight is held up by gas pressure. Do stars exist that are held up by electron degeneracy pressure, rather than gas pressure? • Yes: white dwarfs. How are such stars made? • From normal stars at the end of life, when they have run out of fuel, can’t gen ...
... Most stable stars are stable because their weight is held up by gas pressure. Do stars exist that are held up by electron degeneracy pressure, rather than gas pressure? • Yes: white dwarfs. How are such stars made? • From normal stars at the end of life, when they have run out of fuel, can’t gen ...
Compact stars
... high temperatures to maintain their pressure. Barring external perturbation or baryon decay, they will persist virtually forever, although black holes are generally believed to finally evaporate from Hawking radiation. Eventually, given enough time (when we enter the so-called degenerate era of the ...
... high temperatures to maintain their pressure. Barring external perturbation or baryon decay, they will persist virtually forever, although black holes are generally believed to finally evaporate from Hawking radiation. Eventually, given enough time (when we enter the so-called degenerate era of the ...
ASTR100 Class 01 - University of Maryland Department of
... What would happen to a protostar that formed without any rotation at all? A. Its jets would go in multiple directions. B. It would not have planets. C. It would be very bright in the infrared. D. It would not be round. ...
... What would happen to a protostar that formed without any rotation at all? A. Its jets would go in multiple directions. B. It would not have planets. C. It would be very bright in the infrared. D. It would not be round. ...
1) Which of the following correctly lists the atoms in order of
... a) The pressure increases least for the addition of 0.100 mol H2. b) The pressure increases more for the addition of 0.100 mol CO2 than for the addition of the same amount of N2. c) The increase in pressure will be proportional to the moles of gas added. d) The increase in pressure will be proportio ...
... a) The pressure increases least for the addition of 0.100 mol H2. b) The pressure increases more for the addition of 0.100 mol CO2 than for the addition of the same amount of N2. c) The increase in pressure will be proportional to the moles of gas added. d) The increase in pressure will be proportio ...
PROPERTIES_OF_MATTER
... chemical bonds. – Examples of chemical bonds: • Ionic bond (electrostatic attraction that binds oppositely charged ions) – Usually composed of metal and nonmetal atoms ...
... chemical bonds. – Examples of chemical bonds: • Ionic bond (electrostatic attraction that binds oppositely charged ions) – Usually composed of metal and nonmetal atoms ...
AST101 Lecture 13 The Lives of the Stars
... • Mass < 0.076 M (80 Jovian masses) • Core becomes degenerate before TC reaches H ignition temperature • No stable H burning • Just cool off • Radius ~ Jovian radius ...
... • Mass < 0.076 M (80 Jovian masses) • Core becomes degenerate before TC reaches H ignition temperature • No stable H burning • Just cool off • Radius ~ Jovian radius ...
AST101_lect_13
... • Mass < 0.076 M (80 Jovian masses) • Core becomes degenerate before TC reaches H ignition temperature • No stable H burning • Just cool off • Radius ~ Jovian radius ...
... • Mass < 0.076 M (80 Jovian masses) • Core becomes degenerate before TC reaches H ignition temperature • No stable H burning • Just cool off • Radius ~ Jovian radius ...
Investigating Matter Notes
... KINETIC MOLECULAR THEORY Explains what happens to matter as the _____________________ of the particles _________________ or ___________________ _________________________________ ...
... KINETIC MOLECULAR THEORY Explains what happens to matter as the _____________________ of the particles _________________ or ___________________ _________________________________ ...
AP Chemistry Jeopardy
... Why is the P-Cl bond in PCl5 a stronger bond than the C-O in carbon monoxide? A) Increased Dipole - Dipole Forces B) Hydrogen Bonding ...
... Why is the P-Cl bond in PCl5 a stronger bond than the C-O in carbon monoxide? A) Increased Dipole - Dipole Forces B) Hydrogen Bonding ...
Basic Chemistry Notes II
... 3. The atomic number is the number of protons B. Neutrons 1. Found in nucleus 2. No charge 3. Can be found by subtracting the atomic number from the atomic weight C. Electrons 1. Found outside of nucleus in “shells” 2. Have a negative charge 3. Valence electrons – outermost electron shell. Most impo ...
... 3. The atomic number is the number of protons B. Neutrons 1. Found in nucleus 2. No charge 3. Can be found by subtracting the atomic number from the atomic weight C. Electrons 1. Found outside of nucleus in “shells” 2. Have a negative charge 3. Valence electrons – outermost electron shell. Most impo ...
The life cycle of the Sun – HR Diagram
... reaches 108K He burning ignited in the core via triple alpha process. Red Giant has dense core containing most of its mass at ~ 50 x106K, core is so dense electrons become degenerate electron gas (no longer obey perfect gas law and pressure does not increase with temperature) . High thermal conduc ...
... reaches 108K He burning ignited in the core via triple alpha process. Red Giant has dense core containing most of its mass at ~ 50 x106K, core is so dense electrons become degenerate electron gas (no longer obey perfect gas law and pressure does not increase with temperature) . High thermal conduc ...
The nature of matter
... Gay-Lussac and law of combining volumes When gases react at constant temperature and pressure, they combine in volumes that are related to each other as ratios of small whole numbers His experiments with hydrogen and oxygen had implications for the understanding of the atom and the structures o ...
... Gay-Lussac and law of combining volumes When gases react at constant temperature and pressure, they combine in volumes that are related to each other as ratios of small whole numbers His experiments with hydrogen and oxygen had implications for the understanding of the atom and the structures o ...