Episode 21
... Before World War II. A substitute for silk. 5. What was the first organic synthesis? Wöhler's synthesis of urea. 6. What is a hydrocarbon? A compound formed only of hydrogen and carbon atoms. 7. What is meant by an isomer? Two molecules that have the same formula but have different structures. 8. Ho ...
... Before World War II. A substitute for silk. 5. What was the first organic synthesis? Wöhler's synthesis of urea. 6. What is a hydrocarbon? A compound formed only of hydrogen and carbon atoms. 7. What is meant by an isomer? Two molecules that have the same formula but have different structures. 8. Ho ...
Episode 21
... Before World War II. A substitute for silk. 5. What was the first organic synthesis? Wöhler's synthesis of urea. 6. What is a hydrocarbon? A compound formed only of hydrogen and carbon atoms. 7. What is meant by an isomer? Two molecules that have the same formula but have different structures. 8. Ho ...
... Before World War II. A substitute for silk. 5. What was the first organic synthesis? Wöhler's synthesis of urea. 6. What is a hydrocarbon? A compound formed only of hydrogen and carbon atoms. 7. What is meant by an isomer? Two molecules that have the same formula but have different structures. 8. Ho ...
BP208P. PHARMACEUTICAL ORGANIC CHEMISTRY
... 4 Hours / week 1. Systematic qualitative analysis of unknown organic compounds like 1. Preliminary test: Color, odour, aliphatic/aromatic compounds, saturation and unsaturation, etc. 2. Detection of elements like Nitrogen, Sulphur and Halogen by Lassaigne’s test 3. Solubility test 4. Functional grou ...
... 4 Hours / week 1. Systematic qualitative analysis of unknown organic compounds like 1. Preliminary test: Color, odour, aliphatic/aromatic compounds, saturation and unsaturation, etc. 2. Detection of elements like Nitrogen, Sulphur and Halogen by Lassaigne’s test 3. Solubility test 4. Functional grou ...
directed reading a
... a. what colors the atoms are b. how the atoms are connected c. how heavy the atoms are d. what size the atoms are _____ 4. What do the backbones of some compounds have hundreds or thousands of? a. carbon atoms c. structural formulas b. carbon molecules d. acid ions HYDROCARBONS AND OTHER ORGANIC COM ...
... a. what colors the atoms are b. how the atoms are connected c. how heavy the atoms are d. what size the atoms are _____ 4. What do the backbones of some compounds have hundreds or thousands of? a. carbon atoms c. structural formulas b. carbon molecules d. acid ions HYDROCARBONS AND OTHER ORGANIC COM ...
投影片 1
... The positive charge (+) is placed at the carbon attached to the E class function group (e.g.,=O,-OH, -Br) Consonant pattern: Positives charges are placed at carbon atoms bonded to the E class groups ...
... The positive charge (+) is placed at the carbon attached to the E class function group (e.g.,=O,-OH, -Br) Consonant pattern: Positives charges are placed at carbon atoms bonded to the E class groups ...
Worksheet – Alkanes Alkanes are the simplest organic compounds
... In the last (most common) representation only the carbon chain is drawn. Each C is assumed to have 4 bonds to it, but the H are not explicitly written out. The longest continuous C chain determines the root name for the compound. If additional groups are bonded to the main chain, they are assigned a ...
... In the last (most common) representation only the carbon chain is drawn. Each C is assumed to have 4 bonds to it, but the H are not explicitly written out. The longest continuous C chain determines the root name for the compound. If additional groups are bonded to the main chain, they are assigned a ...
F324 summary - Macmillan Academy
... Hydrolysis and degradable polymers • Condensation polymers have chemical groups that are vulnerable to chemical attack from either acids or alkalis – polyesters (ester group) and polyamides (amide group). This process is known as hydrolysis and results in the breakdown of the polymer. • Disposing o ...
... Hydrolysis and degradable polymers • Condensation polymers have chemical groups that are vulnerable to chemical attack from either acids or alkalis – polyesters (ester group) and polyamides (amide group). This process is known as hydrolysis and results in the breakdown of the polymer. • Disposing o ...
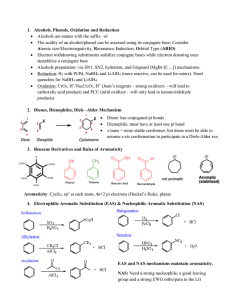
Chem 400 Review Chem 350 JJ.S17
... destabilize a conjugate base Alcohols preparation: via SN1, SN2, hydration, and Grignard (MgBr-[C…]) mechanisms Reduction: H2 with Pt/Pd, NaBH4 and LiAlH4 (more reactive, can be used for esters). Need quenches for NaBH4 and LiAlH4 Oxidation: CrO3, H+/Na2Cr2O7, H+ (Joan’s reagents – strong oxid ...
... destabilize a conjugate base Alcohols preparation: via SN1, SN2, hydration, and Grignard (MgBr-[C…]) mechanisms Reduction: H2 with Pt/Pd, NaBH4 and LiAlH4 (more reactive, can be used for esters). Need quenches for NaBH4 and LiAlH4 Oxidation: CrO3, H+/Na2Cr2O7, H+ (Joan’s reagents – strong oxid ...
Ch 2 BIO Notes
... Thus ions and other polar molecules dissolve easily in water and non-polar molecules like oils float on the surface. ...
... Thus ions and other polar molecules dissolve easily in water and non-polar molecules like oils float on the surface. ...
Elements Found in Living Things
... macromolecule. There are four classes of macromolecules (carbohydrates, lipids, proteins, and nucleic acids). Carbohydrates and lipids are made of only carbon, hydrogen, and oxygen (CHO). Proteins are made of carbon, hydrogen, oxygen, and nitrogen (CHON). Nucleic acids such as DNA and RNA contain ca ...
... macromolecule. There are four classes of macromolecules (carbohydrates, lipids, proteins, and nucleic acids). Carbohydrates and lipids are made of only carbon, hydrogen, and oxygen (CHO). Proteins are made of carbon, hydrogen, oxygen, and nitrogen (CHON). Nucleic acids such as DNA and RNA contain ca ...
Reactions of Alcohols
... SN2 reaction between R-X and R-OWE NEED TO CONSIDER STERIC HINDERANCE. This might lead to E2! ...
... SN2 reaction between R-X and R-OWE NEED TO CONSIDER STERIC HINDERANCE. This might lead to E2! ...
Science 30 Chemistry
... Reactivity- high reactivity similar to alkanes. Within aromatic ring, electrons are shared by all carbons. Responsible for stability. ...
... Reactivity- high reactivity similar to alkanes. Within aromatic ring, electrons are shared by all carbons. Responsible for stability. ...
Class X Chemistry-Carbon and its compounds
... (d) C2H5OH + Na (e) CH3COOH + NaOH 16. An organic compound with molecular formula C2H4O2 produces brisk effervescence on addition of sodium carbonate/bicarbonate. (a) Identify the organic compound. (b) Name the gas evolved. (c) How will you test this gas? (d) Write a chemical equation for the above ...
... (d) C2H5OH + Na (e) CH3COOH + NaOH 16. An organic compound with molecular formula C2H4O2 produces brisk effervescence on addition of sodium carbonate/bicarbonate. (a) Identify the organic compound. (b) Name the gas evolved. (c) How will you test this gas? (d) Write a chemical equation for the above ...
In the preparation of the esters given in this experiment
... the isolation step. Why? What gas was evolved during this washing step? Write a balanced equation for the reaction that produced it. 2. Why is a large excess of acetic acid used in the preparation of isopentyl acetate? 3. Concentrated sulfuric acid is used as a catalyst for the esterification of ace ...
... the isolation step. Why? What gas was evolved during this washing step? Write a balanced equation for the reaction that produced it. 2. Why is a large excess of acetic acid used in the preparation of isopentyl acetate? 3. Concentrated sulfuric acid is used as a catalyst for the esterification of ace ...
Chemistry Lesson 40 Organic Chemistry
... Most organic molecules are bonded to nitrogen or oxygen molecules, along with the carbons and hydrogens. Functional group – group of atoms that determines an organic molecule’s chemical properties. The compounds in each class have similar chemical properties because of the functional groups that the ...
... Most organic molecules are bonded to nitrogen or oxygen molecules, along with the carbons and hydrogens. Functional group – group of atoms that determines an organic molecule’s chemical properties. The compounds in each class have similar chemical properties because of the functional groups that the ...
Organic compounds are covalent compounds composed of carbon
... SPI 0807.9.4 – Differentiate between a mixture and a compound ...
... SPI 0807.9.4 – Differentiate between a mixture and a compound ...
ORGANOMETALLIC COMPOUNDS
... sodium sulphate. Process: chromium trichloride reacts with aluminium and benzene with aluminium chloride as catalyst. The buildet complex then reacts with sodium sulphate and forms Cr(C6H6)2. Electron Counting in organometallic compounds: 18‐electon rule (N.V. Sigwick Rule): by applying this rule ...
... sodium sulphate. Process: chromium trichloride reacts with aluminium and benzene with aluminium chloride as catalyst. The buildet complex then reacts with sodium sulphate and forms Cr(C6H6)2. Electron Counting in organometallic compounds: 18‐electon rule (N.V. Sigwick Rule): by applying this rule ...
.
... . In which of the following compounds would you expect intra-molecular hydrogen bonding to occur? I. o-nitroaniline 11. o-hydroxybenzoic acid (salicyclic acid) 111. o-fluorophenol IV. o-hydrobenzaldehyde (salicyladehyde) (D) I, I11 (A) I, 11, IIIyIV (B) I, 11, IV (C) 11, 111, IV i. Rank the followin ...
... . In which of the following compounds would you expect intra-molecular hydrogen bonding to occur? I. o-nitroaniline 11. o-hydroxybenzoic acid (salicyclic acid) 111. o-fluorophenol IV. o-hydrobenzaldehyde (salicyladehyde) (D) I, I11 (A) I, 11, IIIyIV (B) I, 11, IV (C) 11, 111, IV i. Rank the followin ...
Chemistry_
... Synthetic means artificial (man-made), TeflonTM is an example of another synthetic substance 3. Can you list three properties of KevlarTM that make it so useful? Properties include fire resistance, high tensile strength, lightweight(ness) and flexibility ...
... Synthetic means artificial (man-made), TeflonTM is an example of another synthetic substance 3. Can you list three properties of KevlarTM that make it so useful? Properties include fire resistance, high tensile strength, lightweight(ness) and flexibility ...
Section 7.5~7.6 - www .alexandria .k12 .mn .us
... 1) Why is it important to understand the acid-base behavior of organic molecules? 2) What are the differences in behavior between Lewis acids and Bronsted-Lowry acids? 2.5) In equation 3.53, is the amide ion ( NH2- ) functioning as an acid or a base? 3) How would you explain the concept of pKa to th ...
... 1) Why is it important to understand the acid-base behavior of organic molecules? 2) What are the differences in behavior between Lewis acids and Bronsted-Lowry acids? 2.5) In equation 3.53, is the amide ion ( NH2- ) functioning as an acid or a base? 3) How would you explain the concept of pKa to th ...
Professor Marina Gatti
... aromatic substitution. Monosubstituted benzenes: effect of substituents on substitution rate and orientation. ...
... aromatic substitution. Monosubstituted benzenes: effect of substituents on substitution rate and orientation. ...
phenols - Gneet`s
... In phenols the –OH group is attached to sp2 hybrid carbon of an aromatic ring and the oxygen atom of the hydroxyl group has two lone pairs of electrons and the bond angle in phenol is 109o. The C-O bond length in phenol (136pm) is slightly less than in methanol(412pm) due to resonance in aromatic ri ...
... In phenols the –OH group is attached to sp2 hybrid carbon of an aromatic ring and the oxygen atom of the hydroxyl group has two lone pairs of electrons and the bond angle in phenol is 109o. The C-O bond length in phenol (136pm) is slightly less than in methanol(412pm) due to resonance in aromatic ri ...
rev2
... 7. Know about the structure of phenols. 8. Be able to name phenols 9. Phenols are more soluble in water than alcohols because they are more polar. Know about the chemical reactivity a. Phenols are acidic in water (carbolic acid) b. Be able to finish aromatic reactions with phenols. (They do not act ...
... 7. Know about the structure of phenols. 8. Be able to name phenols 9. Phenols are more soluble in water than alcohols because they are more polar. Know about the chemical reactivity a. Phenols are acidic in water (carbolic acid) b. Be able to finish aromatic reactions with phenols. (They do not act ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.