投影片 1
... a positive charge, whereas the other E class group resides on a carbon with a negative charge. ...
... a positive charge, whereas the other E class group resides on a carbon with a negative charge. ...
Organic Chemistry –Syllabus- one Semester Sackler faculty of
... Organic Compounds + Alkanes double bond equivalent, alkyl group, Nomenclature (IUPAC rules), intermolecular forces( van der Waals force, Dipole–dipole interaction, Hydrogen bonds), Solubility, Conformations of alkanes(staggered-eclipsd) , Cycloalkanes, geometric isomers, The chair conformation of cy ...
... Organic Compounds + Alkanes double bond equivalent, alkyl group, Nomenclature (IUPAC rules), intermolecular forces( van der Waals force, Dipole–dipole interaction, Hydrogen bonds), Solubility, Conformations of alkanes(staggered-eclipsd) , Cycloalkanes, geometric isomers, The chair conformation of cy ...
Chap Thirteen: Alcohols
... iii. reaction with acid chlorides or esters (double addition) iv. reaction with epoxides (Anti stereoselective; SN2-like regioselectivity) v. Side reactions with acidic compounds d. Via reduction of carbonyls or epoxides with Hydride Reducing reagents i. Reduction of Ketones, Aldehydes and Epoxides ...
... iii. reaction with acid chlorides or esters (double addition) iv. reaction with epoxides (Anti stereoselective; SN2-like regioselectivity) v. Side reactions with acidic compounds d. Via reduction of carbonyls or epoxides with Hydride Reducing reagents i. Reduction of Ketones, Aldehydes and Epoxides ...
Organic Chemistry
... • We have stressed throughout the text that the synthesis of chiral products from achiral starting materials and under achiral reaction conditions of necessity gives enantiomers as a racemic mixture. • Nature achieves the synthesis of single enantiomers by using enzymes, which create a chiral enviro ...
... • We have stressed throughout the text that the synthesis of chiral products from achiral starting materials and under achiral reaction conditions of necessity gives enantiomers as a racemic mixture. • Nature achieves the synthesis of single enantiomers by using enzymes, which create a chiral enviro ...
organic lab questions
... What is responsible for the brown colour in the bromine water? What is the concentration of bromine in the bromine water (give an approximate percentage and please provide a reference for where you have found this infomration). If the brown colour fades to clear, what precisely has happened to the b ...
... What is responsible for the brown colour in the bromine water? What is the concentration of bromine in the bromine water (give an approximate percentage and please provide a reference for where you have found this infomration). If the brown colour fades to clear, what precisely has happened to the b ...
- professional publication
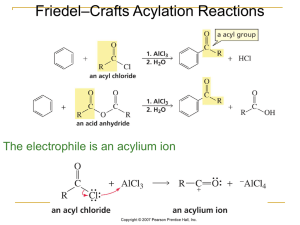
... Electrophilic Aromatic Substitutions Effect of Substituent Groups, Determination of Orientation, Determination of Relative Reactivity, Classification of Substituent Groups, Mechanism of Nitration, Sulphonation, Halogenation, Friedel Craft’s Alkylation and Friedel Craft’s Acylation, Reactivity and Or ...
... Electrophilic Aromatic Substitutions Effect of Substituent Groups, Determination of Orientation, Determination of Relative Reactivity, Classification of Substituent Groups, Mechanism of Nitration, Sulphonation, Halogenation, Friedel Craft’s Alkylation and Friedel Craft’s Acylation, Reactivity and Or ...
Chemistry 218, Winter 2007 Exam 2 Name: 1.
... 2. In the following reaction, one of the products is formed preferentially over the other one. Circle the product that is more likely to be formed, and explain why using resonance forms. (10 pts) O ...
... 2. In the following reaction, one of the products is formed preferentially over the other one. Circle the product that is more likely to be formed, and explain why using resonance forms. (10 pts) O ...
Unit 3 Goals - kimscience.com
... Go over your notes from class and from reading and reorganize them – turn tables or diagrams into paragraph explanations and vice versa. Identify cause and effect whenever possible. Make a concept map using the above vocabulary terms, connecting them as fully as possible. Make sure you can fully exp ...
... Go over your notes from class and from reading and reorganize them – turn tables or diagrams into paragraph explanations and vice versa. Identify cause and effect whenever possible. Make a concept map using the above vocabulary terms, connecting them as fully as possible. Make sure you can fully exp ...
CHAPTER-6 DEHYDROHALOGENATION OF ALKYL HALIDES
... Dehydration of Alcohols to form Ethers • Simple, symmetrical ethers can be formed from the intermolecular acid‐catalyzed dehydration of 1° (or methyl) alcohols (a “substitution reaction”) • 2° and 3° alcohols can’t be used because they eliminate (intramolecular dehydration) to form alkenes ...
... Dehydration of Alcohols to form Ethers • Simple, symmetrical ethers can be formed from the intermolecular acid‐catalyzed dehydration of 1° (or methyl) alcohols (a “substitution reaction”) • 2° and 3° alcohols can’t be used because they eliminate (intramolecular dehydration) to form alkenes ...
What is an addition reaction
... Halogenatated alkane Halogen Halogenation Double Halogenated alkane Condensation (also called Elimination) In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must ha ...
... Halogenatated alkane Halogen Halogenation Double Halogenated alkane Condensation (also called Elimination) In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must ha ...
Applications of Phosphorus, Sulfur, Silicon and Boron Chemistry:
... Formulate the P, S or Si product formed from a given set of reagents (as covered in the course), e.g. synthesis of phosphonates, phosphonium salts, ...
... Formulate the P, S or Si product formed from a given set of reagents (as covered in the course), e.g. synthesis of phosphonates, phosphonium salts, ...
Etherification of monosaccharide with isobutene: A - chem
... some bio-feedstock compounds. A recent example is etherification of glycerol and ethylene glycol with isobutene over acidic catalysts. [1] [2] The reaction is straightforward thanks to the facile formation of tertiary carbocation from isobutene, which subsequently reacts with the alcohols. ...
... some bio-feedstock compounds. A recent example is etherification of glycerol and ethylene glycol with isobutene over acidic catalysts. [1] [2] The reaction is straightforward thanks to the facile formation of tertiary carbocation from isobutene, which subsequently reacts with the alcohols. ...
Final Exam Review Sheet Chemistry 110a/1998
... The final exam questions will seek an integrated understanding of the material found in chapters 1-13. You will be allowed to use the following when working your final exam: a calculator, molecular models, 13 pieces of unlined white 8.5 x 11 inch paper on which you may hand-write any information to ...
... The final exam questions will seek an integrated understanding of the material found in chapters 1-13. You will be allowed to use the following when working your final exam: a calculator, molecular models, 13 pieces of unlined white 8.5 x 11 inch paper on which you may hand-write any information to ...
Chem 2641 Chapter 5 Understanding Organic Reactions I. Writing
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
CHE 322
... a. 2-heptanone from simple alcohols of 4 C or fewer. No strong base like LDA is available. (10 pt) ...
... a. 2-heptanone from simple alcohols of 4 C or fewer. No strong base like LDA is available. (10 pt) ...
Solution Key - Chemistry With BT
... Is the stereoisomer obtained in the reaction above optically active? Explain. No, it is not possible to obtain a chiral product from an achiral reactant unless chiral reaction conditions are utilized, such as enzyme catalysis ...
... Is the stereoisomer obtained in the reaction above optically active? Explain. No, it is not possible to obtain a chiral product from an achiral reactant unless chiral reaction conditions are utilized, such as enzyme catalysis ...
Exam - Chemistry With BT
... requires more than one step. Show all the steps of the synthesis in the right sequence. Give the reagents used and the reaction conditions utilized (including acid base catalysis). Show the structures of all intermediate products. ...
... requires more than one step. Show all the steps of the synthesis in the right sequence. Give the reagents used and the reaction conditions utilized (including acid base catalysis). Show the structures of all intermediate products. ...
DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS
... On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the f ...
... On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the f ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... Write the Woodward Hoffmann rules for cycloaddition reactions. What is group transfer reaction? Give an example. Give the (1,5)-sigmatropic rearrangement reactions. What is photoisomerisation? Draw Jablonskii diagram and mention the photophysical processes. Mention the importance of retrosynthetic a ...
... Write the Woodward Hoffmann rules for cycloaddition reactions. What is group transfer reaction? Give an example. Give the (1,5)-sigmatropic rearrangement reactions. What is photoisomerisation? Draw Jablonskii diagram and mention the photophysical processes. Mention the importance of retrosynthetic a ...
Combustion, Addition and Elimination Objective Combustion Example
... combustion of 2-methypentane. ...
... combustion of 2-methypentane. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... 01. What are the effects of reagents and solvents in Stobbe reaction? Give examples. 02. How is carbene synthesized? In what form it is used in reactions? Give an example. 03. Identify any two types of 1,3-dipolar compounds used for cycloaddition reactions. Give an example. ...
... 01. What are the effects of reagents and solvents in Stobbe reaction? Give examples. 02. How is carbene synthesized? In what form it is used in reactions? Give an example. 03. Identify any two types of 1,3-dipolar compounds used for cycloaddition reactions. Give an example. ...
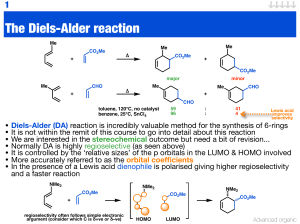
Diels–Alder reaction
.png?width=300)
The Diels–Alder reaction is an organic chemical reaction (specifically, a [4+2] cycloaddition) between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. It was first described by Otto Paul Hermann Diels and Kurt Alder in 1928, for which work they were awarded the Nobel Prize in Chemistry in 1950. The Diels–Alder reaction is particularly useful in synthetic organic chemistry as a reliable method for forming 6-membered systems with good control over regio- and stereochemical properties. The underlying concept has also been applied to other π-systems, such as carbonyls and imines, to furnish the corresponding heterocycles, known as the hetero-Diels–Alder reaction. Diels–Alder reactions can be reversible under certain conditions; the reverse reaction is known as the retro-Diels–Alder reaction.