Jeopardy
... What is the name of the rule that states that the nucleophilic part of the reagent will bind with the most stable carbon of the substrate? ...
... What is the name of the rule that states that the nucleophilic part of the reagent will bind with the most stable carbon of the substrate? ...
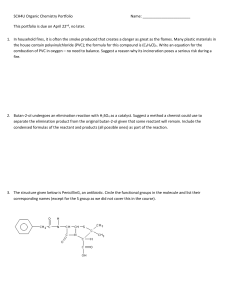
SCH4U Organic Chemistry Portfolio Name: This portfolio is due on
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
... 5. Nomex is a polymer used to make flame-resistant clothing for firefighters. A portion of its structure is provided below. Write a polymerization reaction showing its production from monomers. What type of reaction is this? ...
Enantiospecific skeleton expanding cross
... with an extremely cheap ZnCl2 catalyst, and high enantiospecificity render this reaction ideal for low cost organic synthesis. In just one example, a lactic acid derivative is converted to 2-methylhexanoic acid with excellent yield. This compound is an important building block in the production of t ...
... with an extremely cheap ZnCl2 catalyst, and high enantiospecificity render this reaction ideal for low cost organic synthesis. In just one example, a lactic acid derivative is converted to 2-methylhexanoic acid with excellent yield. This compound is an important building block in the production of t ...
Lab 2 - Academic Computer Center
... How does one recognize a Diels-Alder reaction? These reactions are easy to spot, because they involve two organic reactants and only heat as a reactant. One of the reactants (the diene) must have a conjugated diene system, and the other reactant (the dienophile) must contain a double bond or triple ...
... How does one recognize a Diels-Alder reaction? These reactions are easy to spot, because they involve two organic reactants and only heat as a reactant. One of the reactants (the diene) must have a conjugated diene system, and the other reactant (the dienophile) must contain a double bond or triple ...
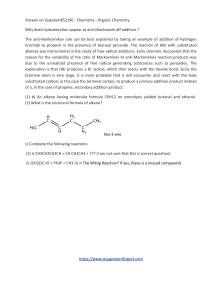
Answer on Question#52196 - Chemistry
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination ...
... Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination ...
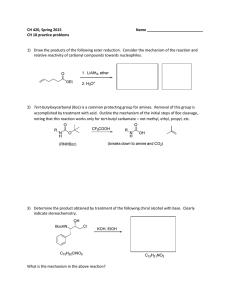
CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
... 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ...
... 7) Rank the following compounds according to their relative acidity: cyclohexanol, phenol, pmethoxyphenol, p-nitrophenol. ...
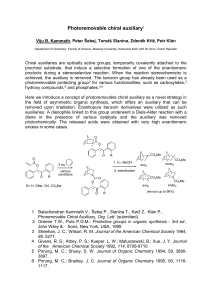
Viju B - IS MU
... prochiral substrate, that induce a selective formation of one of the enantiomeric products during a stereoselective reaction. When the reaction stereochemistry is achieved, the auxiliary is removed. The benzoin group has already been used as a photoremovable protecting group2 for various functionali ...
... prochiral substrate, that induce a selective formation of one of the enantiomeric products during a stereoselective reaction. When the reaction stereochemistry is achieved, the auxiliary is removed. The benzoin group has already been used as a photoremovable protecting group2 for various functionali ...
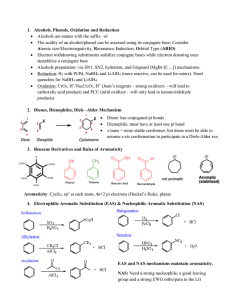
Chem 400 Review Chem 350 JJ.S17
... carboxylic acid product) and PCC (mild oxidizer – will only lead to ketone/aldehyde products) 2. Dienes, Dienophiles, Diels –Alder Mechanism ...
... carboxylic acid product) and PCC (mild oxidizer – will only lead to ketone/aldehyde products) 2. Dienes, Dienophiles, Diels –Alder Mechanism ...
Chapter 7: Dienes
... …that the pi-bonding electrons rearrange themselves into new bonds, joining the diene and the dienophile together in a pericyclic, concerted reaction! The Diels-Alder cycloaddition results in 2 new σ bonds, with one π bond moving to a new position occurs when a diene meets an alkene or alkyne, p ...
... …that the pi-bonding electrons rearrange themselves into new bonds, joining the diene and the dienophile together in a pericyclic, concerted reaction! The Diels-Alder cycloaddition results in 2 new σ bonds, with one π bond moving to a new position occurs when a diene meets an alkene or alkyne, p ...
Assignment 2 Group A and B
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
Chapter 7: Alkenes and Alkynes – Properties and Synthesis
... Allylic bromination, N-Bromosuccinimide MO of allyl radical and allyl cation Rules for writing resonance structures ...
... Allylic bromination, N-Bromosuccinimide MO of allyl radical and allyl cation Rules for writing resonance structures ...
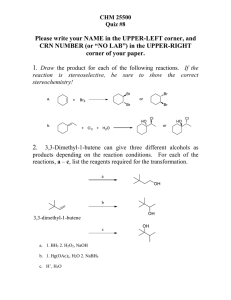
CHEM 201 Name Quiz 10 (Ch 17) ID Q1. Which of the following
... Q3. Which of the following reactions would not normally yield an alcohol? a) Oxymercuration/ demercuraction of ...
... Q3. Which of the following reactions would not normally yield an alcohol? a) Oxymercuration/ demercuraction of ...
$doc.title
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
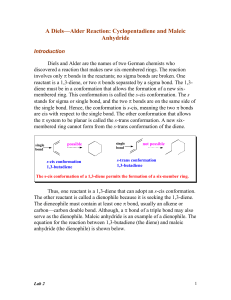
A Diels-Alder Synthesis
... The Diels-Alder reaction usually proceeds with endo selectivity. This means that the product in which the activating electron-withdrawing group of the dienophile is located in the endo position is formed faster than the alternative exo isomer. This happens even though the exo product is sometimes mo ...
... The Diels-Alder reaction usually proceeds with endo selectivity. This means that the product in which the activating electron-withdrawing group of the dienophile is located in the endo position is formed faster than the alternative exo isomer. This happens even though the exo product is sometimes mo ...
Diels-Alder Reaction:
... Even though s-trans conformation of dienes is more stable due to steric reasons, the s-cis conformation is needed to carry out the Diels-Alder reaction. The s-cis conformation of the diene would yield a six membered ring with a cis double bond whereas a diene in s-trans conformation would demand a t ...
... Even though s-trans conformation of dienes is more stable due to steric reasons, the s-cis conformation is needed to carry out the Diels-Alder reaction. The s-cis conformation of the diene would yield a six membered ring with a cis double bond whereas a diene in s-trans conformation would demand a t ...
Diels-Alder Reaction
... without the intervention of radicals, carbocations, or other intermediates. It is a powerful method for the construction of cyclohexene rings, and has become such a mainstay of organic synthesis that its discoverers, Otto Diels and Kurt Alder, were awarded the Nobel Prize in Chemistry in 1950 for th ...
... without the intervention of radicals, carbocations, or other intermediates. It is a powerful method for the construction of cyclohexene rings, and has become such a mainstay of organic synthesis that its discoverers, Otto Diels and Kurt Alder, were awarded the Nobel Prize in Chemistry in 1950 for th ...
Diels–Alder reaction
.png?width=300)
The Diels–Alder reaction is an organic chemical reaction (specifically, a [4+2] cycloaddition) between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. It was first described by Otto Paul Hermann Diels and Kurt Alder in 1928, for which work they were awarded the Nobel Prize in Chemistry in 1950. The Diels–Alder reaction is particularly useful in synthetic organic chemistry as a reliable method for forming 6-membered systems with good control over regio- and stereochemical properties. The underlying concept has also been applied to other π-systems, such as carbonyls and imines, to furnish the corresponding heterocycles, known as the hetero-Diels–Alder reaction. Diels–Alder reactions can be reversible under certain conditions; the reverse reaction is known as the retro-Diels–Alder reaction.