Aldehydes and Ketones
... When a secondary amine reacts with an aldehyde or ketone, there is only one hydrogen that can come off of the nitrogen to form water. The second hydrogen comes off of a neighboring carbon to the carbonyl – the α-carbon – to form an enamine product. When the alkene forms, the Zaitsev alkene when it c ...
... When a secondary amine reacts with an aldehyde or ketone, there is only one hydrogen that can come off of the nitrogen to form water. The second hydrogen comes off of a neighboring carbon to the carbonyl – the α-carbon – to form an enamine product. When the alkene forms, the Zaitsev alkene when it c ...
19.19 Summary
... polar and have higher boiling points than alkanes of comparable size and shape. Esters don’t form hydrogen bonds to other ester molecules so have lower boiling points than analogous alcohols. They can form hydrogen bonds to water and so are comparable to alcohols in their solubility in water. ...
... polar and have higher boiling points than alkanes of comparable size and shape. Esters don’t form hydrogen bonds to other ester molecules so have lower boiling points than analogous alcohols. They can form hydrogen bonds to water and so are comparable to alcohols in their solubility in water. ...
Common aldehydes and ketones
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
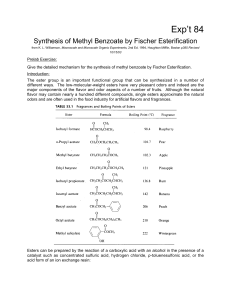
Synthesis of Methyl Benzoate by Fisher Esterification
... concentration of either the alcohol or acid, as noted above. If either one is doubled, the theoretical yield increases to 85%. When one is tripled, it goes to 90%. But note that in the example cited the boiling point of the relatively nonpolar ester is only about 8°C higher than the boiling points o ...
... concentration of either the alcohol or acid, as noted above. If either one is doubled, the theoretical yield increases to 85%. When one is tripled, it goes to 90%. But note that in the example cited the boiling point of the relatively nonpolar ester is only about 8°C higher than the boiling points o ...
Lecture 7a
... The situation changes in the protonated form of the carboxylic acid in which the carbonyl carbon bears a larger positive charge (~0.2 units in the case of acetic acid), which makes it a better electrophile ...
... The situation changes in the protonated form of the carboxylic acid in which the carbonyl carbon bears a larger positive charge (~0.2 units in the case of acetic acid), which makes it a better electrophile ...
A Diels-Alder Synthesis
... So, our Diels-Alder synthesis of cis-Norbornene-5,6-endo-Dicarboxylic Anhydride will first involve the "cracking" of the Dicyclopentadiene into Cyclopentadiene. This product will then be immediately treated with Maleic Anhydride to carry-out our desired DielsAlder reaction. ...
... So, our Diels-Alder synthesis of cis-Norbornene-5,6-endo-Dicarboxylic Anhydride will first involve the "cracking" of the Dicyclopentadiene into Cyclopentadiene. This product will then be immediately treated with Maleic Anhydride to carry-out our desired DielsAlder reaction. ...
Fisher Esterification - OpenBU
... mL of concentrated sulfuric acid and immediately swirl the solution. (CAUTION: Do not reverse the order of this addition. Always add sulfuric acid to the solvent mixture to avoid splattering!) Secure the screw cap firmly over the top of the vessel (make sure your stirrer is inside!) and place your s ...
... mL of concentrated sulfuric acid and immediately swirl the solution. (CAUTION: Do not reverse the order of this addition. Always add sulfuric acid to the solvent mixture to avoid splattering!) Secure the screw cap firmly over the top of the vessel (make sure your stirrer is inside!) and place your s ...
Chemdraw B&W - Pennsylvania State University
... are mirror images and are equal in energy • However, if the reaction is subject to catalysis, a chiral catalyst can create a lower energy pathway for one enantiomer - called an enantionselective synthesis • Reaction of benzaldehyde with diethylzinc with a chiral titanium-containing catalyst, gives 9 ...
... are mirror images and are equal in energy • However, if the reaction is subject to catalysis, a chiral catalyst can create a lower energy pathway for one enantiomer - called an enantionselective synthesis • Reaction of benzaldehyde with diethylzinc with a chiral titanium-containing catalyst, gives 9 ...
Organic Reactions
... b. many organic reactions happen through the attraction of electrophiles for nucleophiles c. in reaction mechanisms, curly arrows show how electrons move – generally electrons from nucleophile move to electrophile 3. Alkanes are relatively inert compared to other functional groups a. Alkenes have pi ...
... b. many organic reactions happen through the attraction of electrophiles for nucleophiles c. in reaction mechanisms, curly arrows show how electrons move – generally electrons from nucleophile move to electrophile 3. Alkanes are relatively inert compared to other functional groups a. Alkenes have pi ...
6.1.3 revision guide carboxylic acids and esters
... CH3CH2CO2CH3 + NaOH CH3CH2CO2- Na+ + CH3OH methyl propanoate sodium propanoate methanol The carboxylic acid salt product is the anion of the carboxylic acid. The anion is resistant to attack by weak nucleophiles such as alcohols, so the reaction is not reversible. ...
... CH3CH2CO2CH3 + NaOH CH3CH2CO2- Na+ + CH3OH methyl propanoate sodium propanoate methanol The carboxylic acid salt product is the anion of the carboxylic acid. The anion is resistant to attack by weak nucleophiles such as alcohols, so the reaction is not reversible. ...
Solid phase reactions II
... (e.g. active esters, anhydrides) best conditions highly dependent on the steric and electronic nature of amine and carboxylic acid ...
... (e.g. active esters, anhydrides) best conditions highly dependent on the steric and electronic nature of amine and carboxylic acid ...
Aldehydes Ketones
... Relative Reactivity of Aldehydes & Ketones Aliphatic aldehydes >>> Aromatic aldehydes The electon-donating resonance effect of the aromatic ring makes the carbonyl group less electrophilic than the carbonyl group of the aliphatic aldehyde. ...
... Relative Reactivity of Aldehydes & Ketones Aliphatic aldehydes >>> Aromatic aldehydes The electon-donating resonance effect of the aromatic ring makes the carbonyl group less electrophilic than the carbonyl group of the aliphatic aldehyde. ...
Chapter 21 The Chemistry of Carboxylic Acid Derivatives
... insufficiently acidic to effect protonation of the —OH group of the alcohol. This protonation is necessary to convert this group into a good leaving group. Furthermore, there is virtually no cyanide ion (–C'N) present, and hence virtually no nucleophile to displace the —OH group. First of all, an ex ...
... insufficiently acidic to effect protonation of the —OH group of the alcohol. This protonation is necessary to convert this group into a good leaving group. Furthermore, there is virtually no cyanide ion (–C'N) present, and hence virtually no nucleophile to displace the —OH group. First of all, an ex ...
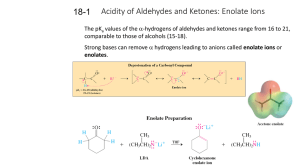
18-1 Enolates (PPT)
... Either equilibration is fast and reversible in solution in the presence of the required catalyst. ...
... Either equilibration is fast and reversible in solution in the presence of the required catalyst. ...
Organic Pathways
... liquids are separated by what can be considered to be a succession of simple distillations. • When the mixture of liquids is heated in the distillation flask, the vapours rise up the fractioning column. • These vapours contain a higher concentration of the more volatile component than the liquid in ...
... liquids are separated by what can be considered to be a succession of simple distillations. • When the mixture of liquids is heated in the distillation flask, the vapours rise up the fractioning column. • These vapours contain a higher concentration of the more volatile component than the liquid in ...
Chapter 7 Alkenes and Alkynes I
... If the group of highest priority on one carbon is on the same side as the group of highest priority on the other carbon the double bond is Z (zusammen) If the highest priority groups are on opposite sides the alkene is E (entgegen) ...
... If the group of highest priority on one carbon is on the same side as the group of highest priority on the other carbon the double bond is Z (zusammen) If the highest priority groups are on opposite sides the alkene is E (entgegen) ...
Aldehydes and Ketones
... alcohol can set-up a better leaving group. Protonation of a carbonyl can create a ...
... alcohol can set-up a better leaving group. Protonation of a carbonyl can create a ...
SORAN UNIVERSITY
... alkene, etc. and how can the students differentiated between these organic families, by understanding their (nomenclatures, properties, and their general reactions). Also the students can learns the basic principal about the some important mechanism for the reactions of these organic compounds. On t ...
... alkene, etc. and how can the students differentiated between these organic families, by understanding their (nomenclatures, properties, and their general reactions). Also the students can learns the basic principal about the some important mechanism for the reactions of these organic compounds. On t ...
Esters
... esterification is also known as ethanoylation. • Ethanoic anhydride is a more vigourous ethanoylating agent ...
... esterification is also known as ethanoylation. • Ethanoic anhydride is a more vigourous ethanoylating agent ...
Octenes from E1 versus E2 Eliminations
... Fill a 10 x 100 mm reaction tube to the 0.5 mL mark with 1-octanol (n-octyl alcohol) and insert a 1/2-inch stir bar. Add 5 drops of conc. sulfuric acid. While stirring, heat the reaction for 20 to 30 minutes. At first you will see water droplets and a cloudy liquid condensing on the walls of the rea ...
... Fill a 10 x 100 mm reaction tube to the 0.5 mL mark with 1-octanol (n-octyl alcohol) and insert a 1/2-inch stir bar. Add 5 drops of conc. sulfuric acid. While stirring, heat the reaction for 20 to 30 minutes. At first you will see water droplets and a cloudy liquid condensing on the walls of the rea ...
Rxns of Alkynes
... 3. if 2 triple bonds, diyne a. if 3 trple bonds, triyne b. if 4, tetrayne, then pentayne, hexayne, heptayne, etc 4. with number (#) as prefix a. funct grp has priority with numbering 5. if more than 1 subst., place all in alphabetical order in front 6. if counting from either end is tie in yne #, us ...
... 3. if 2 triple bonds, diyne a. if 3 trple bonds, triyne b. if 4, tetrayne, then pentayne, hexayne, heptayne, etc 4. with number (#) as prefix a. funct grp has priority with numbering 5. if more than 1 subst., place all in alphabetical order in front 6. if counting from either end is tie in yne #, us ...
WRL0437.tmp
... Alkyl halides are most commonly synthesized from alcohols by replacing the hydroxyl group with a halide substituent. This is an example of nucleophilic aliphatic substitution, which is part of a very important group of reactions. The overall reaction is the same, but the mechanism varies depending o ...
... Alkyl halides are most commonly synthesized from alcohols by replacing the hydroxyl group with a halide substituent. This is an example of nucleophilic aliphatic substitution, which is part of a very important group of reactions. The overall reaction is the same, but the mechanism varies depending o ...
ch16 by dr. Dina
... Dissolving aldehydes (or ketones) in water causes formation of an equilibrium between the carbonyl compound and its hydrate The hydrate is also called a gem-diol (gem i.e. geminal, indicates the presence of two identical substituents on the same carbon) The equilibrum favors a ketone over its hyd ...
... Dissolving aldehydes (or ketones) in water causes formation of an equilibrium between the carbonyl compound and its hydrate The hydrate is also called a gem-diol (gem i.e. geminal, indicates the presence of two identical substituents on the same carbon) The equilibrum favors a ketone over its hyd ...
level three chemistry: organics
... I can show understanding of organic reactions by confidently being able to describe how to use a range of reagents to distinguish between given substances based on observations including colour, separation, bubbles, reactivity, smell etc. I can show my ability to link my understanding of different ...
... I can show understanding of organic reactions by confidently being able to describe how to use a range of reagents to distinguish between given substances based on observations including colour, separation, bubbles, reactivity, smell etc. I can show my ability to link my understanding of different ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.