WRL3502.tmp
... General Concepts Alkyl halides are most commonly synthesized from alcohols by replacing the hydroxyl group with a halide substituent. This is an example of nucleophilic aliphatic substitution, which is part of a very important group of reactions. The overall reaction is the same, but the mechanism v ...
... General Concepts Alkyl halides are most commonly synthesized from alcohols by replacing the hydroxyl group with a halide substituent. This is an example of nucleophilic aliphatic substitution, which is part of a very important group of reactions. The overall reaction is the same, but the mechanism v ...
Spring 2015 CH 421 Name ________________________________________ Section ___________ Post‐lab 3: The Grignard Reaction: Preparation of an Alcohol
... 4) Aldehydes undergo reaction with a Grignard reagent to provide an alcohol product. Many aldehydes are prone to air oxidation. For instance, a bottle of benzaldehyde will turn from a clear liquid to a white solid if left open over time. What is the oxidation produ ...
... 4) Aldehydes undergo reaction with a Grignard reagent to provide an alcohol product. Many aldehydes are prone to air oxidation. For instance, a bottle of benzaldehyde will turn from a clear liquid to a white solid if left open over time. What is the oxidation produ ...
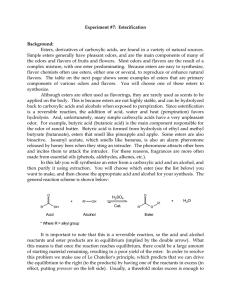
102 Lab 7 Esters Fall05
... flavor chemists often use esters, either one or several, to reproduce or enhance natural flavors. The table on the next page shows some examples of esters that are primary components of various odors and flavors. You will choose one of these esters to synthesize. Although esters are often used as fl ...
... flavor chemists often use esters, either one or several, to reproduce or enhance natural flavors. The table on the next page shows some examples of esters that are primary components of various odors and flavors. You will choose one of these esters to synthesize. Although esters are often used as fl ...
1072. A General Synthesis of Ethers.
... of the reaction mixtures was carried out with a,n Aerograph dual-column temperature-programming gas chromatograph, model A-350-B, from Wilkens Instrument & Research, Inc., Berkely, Calif. The columns (3 m.) were filled with Chromosorb W coated with 15% of Silicone oil 550 or Apiezon L. Analysis was ...
... of the reaction mixtures was carried out with a,n Aerograph dual-column temperature-programming gas chromatograph, model A-350-B, from Wilkens Instrument & Research, Inc., Berkely, Calif. The columns (3 m.) were filled with Chromosorb W coated with 15% of Silicone oil 550 or Apiezon L. Analysis was ...
Secondary alcohols
... Rearrangement of an initial secondary carbocation to the more stable tertiary carbocation by a hydride shift results in a rearranged product. ...
... Rearrangement of an initial secondary carbocation to the more stable tertiary carbocation by a hydride shift results in a rearranged product. ...
St.Mont Fort School Bhopal Haloalkanes and Haloarenes Q 1 Give
... 11.Although chlorine is an electron withdrawing group, yet it is ortho-, paradirecting in electrophilic aromatic substitution reactions. Why? 12.The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Expl ...
... 11.Although chlorine is an electron withdrawing group, yet it is ortho-, paradirecting in electrophilic aromatic substitution reactions. Why? 12.The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Expl ...
Exp`t 88 - Chemistry Courses
... but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, hydrolysis of the ester, gives the alcohol and the acid from which it was synthesized. Because the ...
... but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, hydrolysis of the ester, gives the alcohol and the acid from which it was synthesized. Because the ...
Carbonyl Compounds_ Properties and Reactions
... Carbonyls show a limited/lack of hydrogen bonding between molecules, whereas the corresponding alcohol will show extensive intermolecular H bonding. Weaker polarity means aldehydes and ketones mix well with polar solvents such as water and will dissolve many organic compounds. ...
... Carbonyls show a limited/lack of hydrogen bonding between molecules, whereas the corresponding alcohol will show extensive intermolecular H bonding. Weaker polarity means aldehydes and ketones mix well with polar solvents such as water and will dissolve many organic compounds. ...
Topics 10 and 20 Outline
... • Use of partial charges (δ+ and δ-) and wedge-dash three-dimensional representations (using tapered bonds as shown below) should be encouraged where appropriate in explaining reaction mechanisms. ...
... • Use of partial charges (δ+ and δ-) and wedge-dash three-dimensional representations (using tapered bonds as shown below) should be encouraged where appropriate in explaining reaction mechanisms. ...
Carboxylic Acids - University of Nebraska Omaha
... • By careful control of experimental conditions, it is possible to prepare esters in high yield. • If the alcohol is inexpensive relative to the carboxylic acid, it can be used in excess to drive the equilibrium to the right (toward ester ...
... • By careful control of experimental conditions, it is possible to prepare esters in high yield. • If the alcohol is inexpensive relative to the carboxylic acid, it can be used in excess to drive the equilibrium to the right (toward ester ...
THE CARBON-CARBON DOUBLE BOND
... Notes: (1) A primary amide is an amide unsubstituted on the amide nitrogen - amides with alkyl or other substituents on N cannot be dehydrated. (2) SOCl2 is thionyl chloride and POCl3 is phosphorus oxychloride. Both are powerful dehydrating agents. Reactivity of Nitriles: ...
... Notes: (1) A primary amide is an amide unsubstituted on the amide nitrogen - amides with alkyl or other substituents on N cannot be dehydrated. (2) SOCl2 is thionyl chloride and POCl3 is phosphorus oxychloride. Both are powerful dehydrating agents. Reactivity of Nitriles: ...
Nomenclature of Polyfunctional Organic Compounds
... due to the fact that chemical names have more than one purpose. For Chemical Abstracts Service (CAS), which catalogs and indexes the worldwide chemical literature, each compound must have only one correct name. It would be chaos if half the entries for CHsBr were indexed under "M" for methyl bromide ...
... due to the fact that chemical names have more than one purpose. For Chemical Abstracts Service (CAS), which catalogs and indexes the worldwide chemical literature, each compound must have only one correct name. It would be chaos if half the entries for CHsBr were indexed under "M" for methyl bromide ...
A. Acid Halides
... This particular alcoholysis reaction is also called a transesterification reaction because one ester is converted to another ester (useful in converting liquid ester to solid ester; for identification by ...
... This particular alcoholysis reaction is also called a transesterification reaction because one ester is converted to another ester (useful in converting liquid ester to solid ester; for identification by ...
2287 Summary
... Brominated Ketones.-The procedure for the preparation was that of Schmidt,6 according to which the ketone is brominated in glacial acetic acid. a-Bromo-n-va1erophenone.-As this substance has not previously been described in the literature, it may be mentioned that it is a straw-colored liquid boilin ...
... Brominated Ketones.-The procedure for the preparation was that of Schmidt,6 according to which the ketone is brominated in glacial acetic acid. a-Bromo-n-va1erophenone.-As this substance has not previously been described in the literature, it may be mentioned that it is a straw-colored liquid boilin ...
Problems - TigerWeb
... All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is, it is wrong. Where applicable, outline the logic or mystical principle you used to arrive at your answer, as partial credit m ...
... All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer is, it is wrong. Where applicable, outline the logic or mystical principle you used to arrive at your answer, as partial credit m ...
Chapter 11 Carboxylic Anhydrides, Esters, and Amides
... Hydrolysis of Amides Amides require more vigorous conditions for hydrolysis in both acid and base than do esters. • Hydrolysis in hot aqueous acid gives a carboxylic acid and an ammonium ion. • Hydrolysis is driven to completion by the acid-base reaction between ammonia or the amine and the acid to ...
... Hydrolysis of Amides Amides require more vigorous conditions for hydrolysis in both acid and base than do esters. • Hydrolysis in hot aqueous acid gives a carboxylic acid and an ammonium ion. • Hydrolysis is driven to completion by the acid-base reaction between ammonia or the amine and the acid to ...
IB Chemistry HL Assessment Statements 2009 Revised
... Include the identification of the repeating unit. ...
... Include the identification of the repeating unit. ...
Chapter 17
... Thus a variety of alcohols, alkenes, alkyl halides, alkyl substituted aromatic rings, aldehydes can be converted into carboxylic acids ...
... Thus a variety of alcohols, alkenes, alkyl halides, alkyl substituted aromatic rings, aldehydes can be converted into carboxylic acids ...
Chapter 18 - people.vcu.edu
... o Step two: Grignard deprotonates to form the ylide What’s an ylide? An ylide is when you have two atoms next to each other where one has a negative charge, the other has a positive charge, and ...
... o Step two: Grignard deprotonates to form the ylide What’s an ylide? An ylide is when you have two atoms next to each other where one has a negative charge, the other has a positive charge, and ...
Aldehydes and ketones
... • Tautomers are isomers which differ in the placement of: – A hydrogen atom – A double bond – The keto form has a C=O while the enol form has a C=C. ...
... • Tautomers are isomers which differ in the placement of: – A hydrogen atom – A double bond – The keto form has a C=O while the enol form has a C=C. ...
Ethers and Epoxides
... Ethers and Their Relatives • An ether has two organic groups (alkyl, aryl, or vinyl) bonded to the same oxygen atom, R–O–R • Diethyl ether is used industrially as a solvent • Tetrahydrofuran (THF) is a solvent that is a cyclic ether • Thiols (R–S–H) and sulfides (R–S–R) are sulfur (for oxygen) an ...
... Ethers and Their Relatives • An ether has two organic groups (alkyl, aryl, or vinyl) bonded to the same oxygen atom, R–O–R • Diethyl ether is used industrially as a solvent • Tetrahydrofuran (THF) is a solvent that is a cyclic ether • Thiols (R–S–H) and sulfides (R–S–R) are sulfur (for oxygen) an ...
Dissertation:
... Compounds such as hydroxyesters: methyl and ethyl lactate, and alcohols containing chlorine and fluorine (2-chloroethanol and 2,2,2-trifluoroethanol), allyl alcohol having a carbon-carbon double bonds (C=C) and long-chain aliphatic alcohols (C10-C16) were used. It has been shown that in the presence ...
... Compounds such as hydroxyesters: methyl and ethyl lactate, and alcohols containing chlorine and fluorine (2-chloroethanol and 2,2,2-trifluoroethanol), allyl alcohol having a carbon-carbon double bonds (C=C) and long-chain aliphatic alcohols (C10-C16) were used. It has been shown that in the presence ...
Alkene/Alkyne Addition Reactions
... unsymmetrical reagent such as H-Br, H-Cl, or H-OH to an alkene or alkyne is the one obtained when the H atom of the reagent is added to the C atom of the multiple bond that already has the greater number of H atoms. “The rich get richer” ...
... unsymmetrical reagent such as H-Br, H-Cl, or H-OH to an alkene or alkyne is the one obtained when the H atom of the reagent is added to the C atom of the multiple bond that already has the greater number of H atoms. “The rich get richer” ...
Thiobenzoate Photochemistry
... transfer from the amino group in analogy with the reaction of singlet stilbenes. 23 Proton transfer could result in the photoreduction of the thiocarbonyl group. Triphenylamine is a tertiary amine without -C-H bonds. It will reveal what happens in the reaction when the radical is denied a facile a ...
... transfer from the amino group in analogy with the reaction of singlet stilbenes. 23 Proton transfer could result in the photoreduction of the thiocarbonyl group. Triphenylamine is a tertiary amine without -C-H bonds. It will reveal what happens in the reaction when the radical is denied a facile a ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.