PP 04 Atoms_ molecules_ ions
... Periodic Table: Graphic representation of all the elements indicating size, charge, electronic structure, & reactivity (Elements have symbols with 1 upper case letter & maybe a 2nd letter) ...
... Periodic Table: Graphic representation of all the elements indicating size, charge, electronic structure, & reactivity (Elements have symbols with 1 upper case letter & maybe a 2nd letter) ...
Unit 2 Overview
... 2. Recognize that only the number of protons differentiates each element. 3. Discuss the history of the periodic table and its development. 4. Calculate the mass number of an element given the percent abundance and mass of each of the most common isotopes of each element. 5. Use the periodic table t ...
... 2. Recognize that only the number of protons differentiates each element. 3. Discuss the history of the periodic table and its development. 4. Calculate the mass number of an element given the percent abundance and mass of each of the most common isotopes of each element. 5. Use the periodic table t ...
Notes
... -the number of protons in an atom of an element •all atoms of an element have the same atomic # •written as a subscript next to the element’s symbol •in a neutral atom, the number of protons is equal to the number of electrons (balanced charges). ...
... -the number of protons in an atom of an element •all atoms of an element have the same atomic # •written as a subscript next to the element’s symbol •in a neutral atom, the number of protons is equal to the number of electrons (balanced charges). ...
17 review for test - Blair Community Schools
... • who came up with the Plum Pudding model? • who discovered the nucleus? • who came up with the word “atom” meaning • Who put electrons in orbits? indivsible Completion (10) These terms will be used for the completion • Metals • Metalloids • Mass number • Isotopes • Transition elements • Periods • A ...
... • who came up with the Plum Pudding model? • who discovered the nucleus? • who came up with the word “atom” meaning • Who put electrons in orbits? indivsible Completion (10) These terms will be used for the completion • Metals • Metalloids • Mass number • Isotopes • Transition elements • Periods • A ...
Earth`s Chemistry
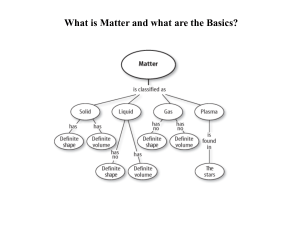
... tightly together in fixed positions Solids have definite shape & volume Liquids = have definite volume but not shape Liquids take the shape of the container Particles tightly packed, but move freely in relation to each other Gases = No definite shape or volume ...
... tightly together in fixed positions Solids have definite shape & volume Liquids = have definite volume but not shape Liquids take the shape of the container Particles tightly packed, but move freely in relation to each other Gases = No definite shape or volume ...
Stable isotope Relative atomic mass Mole fraction Os 183.952 489
... and an equal but opposite (positive) charge. proton – an elementary particle having a rest mass of about 1.673 × 10–27 kg, slightly less than that of a neutron, and a positive electric charge equal and opposite to that of the electron. The number of protons in the nucleus of an atom is the atomic nu ...
... and an equal but opposite (positive) charge. proton – an elementary particle having a rest mass of about 1.673 × 10–27 kg, slightly less than that of a neutron, and a positive electric charge equal and opposite to that of the electron. The number of protons in the nucleus of an atom is the atomic nu ...
Chemistry 1 CP Concept 4 Nuclear Chemistry Study Guide
... 22. Explain what the energy as heat produced by a nuclear power plant is used to ______ _______________________________________________________________________ 23. In nuclear chemistry, an atom is referred to as a(n) ___________________________ 24. Which particle has the same mass as an electron bu ...
... 22. Explain what the energy as heat produced by a nuclear power plant is used to ______ _______________________________________________________________________ 23. In nuclear chemistry, an atom is referred to as a(n) ___________________________ 24. Which particle has the same mass as an electron bu ...
Properties of matter student notes[1]
... Atoms have many parts: Electrons = Invisible, ______________________charged particles. Nucleus = Positively charged _________________________of an atom Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
... Atoms have many parts: Electrons = Invisible, ______________________charged particles. Nucleus = Positively charged _________________________of an atom Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
Radioactivity - Teach Nuclear
... Produced when the nucleus of an atom is in an excited state and then releases energy, becoming more stable When a nucleus emits an or β particle, the daughter nucleus is sometimes left in an excited state. It can then jump down to a lower level by emitting a gamma ray ...
... Produced when the nucleus of an atom is in an excited state and then releases energy, becoming more stable When a nucleus emits an or β particle, the daughter nucleus is sometimes left in an excited state. It can then jump down to a lower level by emitting a gamma ray ...
Protons neutrons electrons Charge Positive neutral negative Mass
... • UPDATE: electrons aren't embedded in positive mass. Atoms' masses exist within the nucleus of the atom - densely packed positively charged center of atoms. Electrons orbit outside nucleus, taking up space ...
... • UPDATE: electrons aren't embedded in positive mass. Atoms' masses exist within the nucleus of the atom - densely packed positively charged center of atoms. Electrons orbit outside nucleus, taking up space ...
Another look at chemical reactions HYDROGEN PEROXIDE WATER
... - ISOTOPES: are atoms of the same element with different mass numbers. In other words, they have the same number of protons but different numbers of neutrons. ...
... - ISOTOPES: are atoms of the same element with different mass numbers. In other words, they have the same number of protons but different numbers of neutrons. ...
Word - The Chemistry Book
... a. Radioactive elements undergo a process of decay over time b. First to artificially transmute one element into another B. Nuclear reactions 1. Involve great quantities of energy Strong nuclear force overcomes electrostatic repulsion between protons 2. Differ from chemical reactions a. atomic numbe ...
... a. Radioactive elements undergo a process of decay over time b. First to artificially transmute one element into another B. Nuclear reactions 1. Involve great quantities of energy Strong nuclear force overcomes electrostatic repulsion between protons 2. Differ from chemical reactions a. atomic numbe ...
History of the Atom and Periodic Table
... Henry Moseley created the Modern Periodic Table of Elements that we use today. It is arranged by atomic number rather than mass. ...
... Henry Moseley created the Modern Periodic Table of Elements that we use today. It is arranged by atomic number rather than mass. ...
The Chemical Basis of Life Chapter 4
... • Elements that make up less than 0.01 percent of you body mass, but are critical to your health. ...
... • Elements that make up less than 0.01 percent of you body mass, but are critical to your health. ...
RAD 354 Chapt 3 Structure of Matter
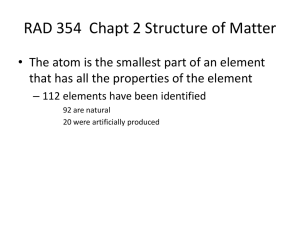
... • The atom is the smallest part of an element that has all the properties of the element – 112 elements have been identified 92 are natural 20 were artificially produced ...
... • The atom is the smallest part of an element that has all the properties of the element – 112 elements have been identified 92 are natural 20 were artificially produced ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.











![Properties of matter student notes[1]](http://s1.studyres.com/store/data/009076956_1-3293fc3fecf578fd34e3f0f2700d471f-300x300.png)