AP Chemistry
... (2) Anions are larger than their parent atoms. (a) Why? More electrons, so more electron-electron repulsions, so electrons spread out more in space. v) For ions carrying the same charge, size increases as we go down a column. (1) Isoelectronic series = group of ions all containing the same number of ...
... (2) Anions are larger than their parent atoms. (a) Why? More electrons, so more electron-electron repulsions, so electrons spread out more in space. v) For ions carrying the same charge, size increases as we go down a column. (1) Isoelectronic series = group of ions all containing the same number of ...
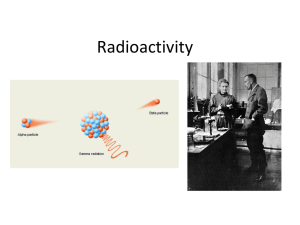
Radioactivity
... • Alpha decay: Mass number ↓4, atomic number ↓2 • Beta minus decay: mass number unchanged, atomic number↑1 • Beta plus decay: mass number unchanged, atomic number ↓1 ...
... • Alpha decay: Mass number ↓4, atomic number ↓2 • Beta minus decay: mass number unchanged, atomic number↑1 • Beta plus decay: mass number unchanged, atomic number ↓1 ...
Chapter 32 Applied Nucleonics
... present in the sample. Quantities as small as 10-9 g of some elements can be detected by neutron activation analysis. ...
... present in the sample. Quantities as small as 10-9 g of some elements can be detected by neutron activation analysis. ...
Chapter 3 Nuclear Radiation
... A chain reaction occurs • when a critical mass of uranium undergoes fission. • releasing a large amount of heat and energy that produces an atomic explosion. Copyright © 2009 by Pearson Education, Inc. ...
... A chain reaction occurs • when a critical mass of uranium undergoes fission. • releasing a large amount of heat and energy that produces an atomic explosion. Copyright © 2009 by Pearson Education, Inc. ...
6.3- periodic trends - burgess
... Electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion Example: F(g) + e- F-(g) Ho (ENERGY) = -328.0 kJ/mol Think of it like electronegativity without the need to bond… It still has to do with attrac ...
... Electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion Example: F(g) + e- F-(g) Ho (ENERGY) = -328.0 kJ/mol Think of it like electronegativity without the need to bond… It still has to do with attrac ...
Periodic Trends
... • Electronegativity increases going left to right across the periodic table. • Fluorine's high nuclear charge coupled with its small size make it hold onto bonding electrons more tightly than any other element. Lithium has a lower nuclear charge and is actually larger than fluorine. Its valence elec ...
... • Electronegativity increases going left to right across the periodic table. • Fluorine's high nuclear charge coupled with its small size make it hold onto bonding electrons more tightly than any other element. Lithium has a lower nuclear charge and is actually larger than fluorine. Its valence elec ...
Atoms1 - Cbsephysicstutorials
... (Hint: The height of the potential barrier is given by the Coulomb repulsion between the two deuterons when they just touch each other. Assume that they can be taken as hard spheres of radius 2.0 fm.) ...
... (Hint: The height of the potential barrier is given by the Coulomb repulsion between the two deuterons when they just touch each other. Assume that they can be taken as hard spheres of radius 2.0 fm.) ...
Chapter NP-4 - NukeWorker.com
... Alpha particles are the most ionizing form of radiation. That is, the specific ionization for alphas is very large. This is due to the fact that the alpha particle has a +2 charge, a large mass and thus a small velocity. An alpha particle forms ion pair when its electric field "pulls" electrons out ...
... Alpha particles are the most ionizing form of radiation. That is, the specific ionization for alphas is very large. This is due to the fact that the alpha particle has a +2 charge, a large mass and thus a small velocity. An alpha particle forms ion pair when its electric field "pulls" electrons out ...
Chapter 18 - An Introduction to Chemistry: Nuclear
... Two forces act upon the particles within the nucleus to produce the nuclear structure. One, called the electrostatic force (or electromagnetic force), is the force that causes opposite electrical charges to attract each other and like charges to repel each other. The positively charged protons in th ...
... Two forces act upon the particles within the nucleus to produce the nuclear structure. One, called the electrostatic force (or electromagnetic force), is the force that causes opposite electrical charges to attract each other and like charges to repel each other. The positively charged protons in th ...
Final Exam Review whole thing
... Protons + with a positive charge Electrons – with a negative charge And Neutrons with a neutral charge ...
... Protons + with a positive charge Electrons – with a negative charge And Neutrons with a neutral charge ...
B - Uplift Education
... Three important components in the design of all nuclear reactors are moderator, control rods and heat exchanger. ▪ Moderator is a medium that slows down fast neutrons to make them suitable for reaction (water, graphite, heavy water). ▪ Control rods are movable rods that readily absorb neutrons. They ...
... Three important components in the design of all nuclear reactors are moderator, control rods and heat exchanger. ▪ Moderator is a medium that slows down fast neutrons to make them suitable for reaction (water, graphite, heavy water). ▪ Control rods are movable rods that readily absorb neutrons. They ...
Matter Unit Study Guide Phases of Matter
... What is the mass of the object on this triple beam balance? ...
... What is the mass of the object on this triple beam balance? ...
50 Frequently Forgotten Facts
... 36) Energy is absorbed to break chemical bonds and released when new bonds are formed. a) Which statement best describes the reaction H + H H2 + energy: 1) A bond is being broken, which absorbs energy 2) A bond is being formed, which absorbs energy 3) A bond is being broken, which releases energy ...
... 36) Energy is absorbed to break chemical bonds and released when new bonds are formed. a) Which statement best describes the reaction H + H H2 + energy: 1) A bond is being broken, which absorbs energy 2) A bond is being formed, which absorbs energy 3) A bond is being broken, which releases energy ...