ANSWERS Using Key Terms Understanding Key Ideas
... exercise can be found at the back of this book. 16. Scientists must determine the atomic number, or the number of protons, in the newly formed nucleus. The nucleus is that of a new element only if the number of protons is different from all known elements. 17. Sample answer: Dalton’s atomic theory w ...
... exercise can be found at the back of this book. 16. Scientists must determine the atomic number, or the number of protons, in the newly formed nucleus. The nucleus is that of a new element only if the number of protons is different from all known elements. 17. Sample answer: Dalton’s atomic theory w ...
The Atom
... atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
... atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
Multiple Choice Questions
... (3) unstable nuclei emit alpha particles (4) unstable nuclei emit beta particles 8. Alpha particles are emitted during the radioactive decay of (1) carbon-14 (2) neon-19 (3) calcium-37 ...
... (3) unstable nuclei emit alpha particles (4) unstable nuclei emit beta particles 8. Alpha particles are emitted during the radioactive decay of (1) carbon-14 (2) neon-19 (3) calcium-37 ...
Chapter 1
... A. Properties of Isotopes B. Telling Isotopes Apart *Notes- The ______mass number_____________ of an atom is the sum of the protons and the neutrons. *Notes-An atom of boron has 5 protons, 6 neutrons, and 5 electrons. It mass number will be _____11_________. (5 protons + 6 neutrons) C. Naming Isotop ...
... A. Properties of Isotopes B. Telling Isotopes Apart *Notes- The ______mass number_____________ of an atom is the sum of the protons and the neutrons. *Notes-An atom of boron has 5 protons, 6 neutrons, and 5 electrons. It mass number will be _____11_________. (5 protons + 6 neutrons) C. Naming Isotop ...
Ch4StudyGuide
... Why do most atoms have no charge even though they are made up of positively charged protons and negatively charged electrons? ...
... Why do most atoms have no charge even though they are made up of positively charged protons and negatively charged electrons? ...
2 C Atomic Number Mass Number Atomic Mass and Isotopes
... Other ways to write elements: Mass Number ...
... Other ways to write elements: Mass Number ...
answers
... d.) Bohr – solar system model of atoms, energy levels at increasing distance from nucleus ...
... d.) Bohr – solar system model of atoms, energy levels at increasing distance from nucleus ...
Foldable - Georgetown ISD
... Ions: when an atom has lost or gained electrons it becomes an ion. Ions have either a positive or negative charge. Atoms do not have a charge because in an ATOM the #protons = #electrons. To calculate the charge of an ion = #protons - #electrons Example: Write the nuclear symbol for an ion with 10 e ...
... Ions: when an atom has lost or gained electrons it becomes an ion. Ions have either a positive or negative charge. Atoms do not have a charge because in an ATOM the #protons = #electrons. To calculate the charge of an ion = #protons - #electrons Example: Write the nuclear symbol for an ion with 10 e ...
Unit 7 Review
... A nucleus of the isotope xenon, Xe-131, is produced when a nucleus of the radioactive isotope iodine I-13 decays. (a) Explain the term isotopes. the nuclei of different isotopes of an element have the same number of protons; but different numbers of neutrons; ...
... A nucleus of the isotope xenon, Xe-131, is produced when a nucleus of the radioactive isotope iodine I-13 decays. (a) Explain the term isotopes. the nuclei of different isotopes of an element have the same number of protons; but different numbers of neutrons; ...
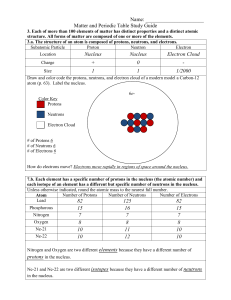
Matter and the Periodic Table Study Guide Answer Key
... Semimetals/Metalloids have properties of both metals and non-metals. 3.b. Compounds are formed by combining two or more different elements and compounds have properties that are different from their constituent elements. 3.f. Use the periodic table to identify elements in simple compounds. Compound ...
... Semimetals/Metalloids have properties of both metals and non-metals. 3.b. Compounds are formed by combining two or more different elements and compounds have properties that are different from their constituent elements. 3.f. Use the periodic table to identify elements in simple compounds. Compound ...
Structure of the Atom - Effingham County Schools
... positive charge to balance the negative electrons. They also showed that atoms must contain other particles that account for most of their mass. ...
... positive charge to balance the negative electrons. They also showed that atoms must contain other particles that account for most of their mass. ...
Chapter Test on 4, 5 2016-2017 _____1. You ar
... 25. In a solution, the part that does the dissolving is called the ___________________ 26. In a solution, the part that gets dissolved is called the ________________________ 27. A mixture where the parts will settle after a while is a ______________________ 28. The atomic mass unit (amu) is the mass ...
... 25. In a solution, the part that does the dissolving is called the ___________________ 26. In a solution, the part that gets dissolved is called the ________________________ 27. A mixture where the parts will settle after a while is a ______________________ 28. The atomic mass unit (amu) is the mass ...
particle - Uplift North Hills
... numbers of protons and neutrons? However, as Z increases the `stability line' curves upwards. Heavier nuclei need more and more neutrons to be stable. Can we explain why? ● It is the strong nuclear force that holds the nucleons together, but this is a very short range force. ● The repulsive electric ...
... numbers of protons and neutrons? However, as Z increases the `stability line' curves upwards. Heavier nuclei need more and more neutrons to be stable. Can we explain why? ● It is the strong nuclear force that holds the nucleons together, but this is a very short range force. ● The repulsive electric ...
Document
... radioactive elements have too many protons or neutrons. Carbon-14, a radioactive form of carbon, has too may neutrons and its nucleus is unstable. This unstable nucleus will vibrate and contort and attempt to become stable by ejecting particles and giving off energy. This is radioactive decay giving ...
... radioactive elements have too many protons or neutrons. Carbon-14, a radioactive form of carbon, has too may neutrons and its nucleus is unstable. This unstable nucleus will vibrate and contort and attempt to become stable by ejecting particles and giving off energy. This is radioactive decay giving ...
Chapter 4 Study Guide-Atomic Structure Define the following terms
... Atomic number-number of protons, periodic table Dalton’s Atomic Theory-first theory to relate chemical changes to events at the atomic level Electron-negatively charged subatomic particle, lives outside of the nucleus Group-vertical column on periodic table Isotopes- atoms of the same element with a ...
... Atomic number-number of protons, periodic table Dalton’s Atomic Theory-first theory to relate chemical changes to events at the atomic level Electron-negatively charged subatomic particle, lives outside of the nucleus Group-vertical column on periodic table Isotopes- atoms of the same element with a ...
ppt-nuclear - SandersScienceStuff
... Fission • Fission means to break apart. Nuclear fission occurs when a nucleus splits apart into different fragments. • This generally occurs with atoms that have a mass number heavier than 60. • The nuclei do not always split the same way. Scientists have found 200 different products from the fissi ...
... Fission • Fission means to break apart. Nuclear fission occurs when a nucleus splits apart into different fragments. • This generally occurs with atoms that have a mass number heavier than 60. • The nuclei do not always split the same way. Scientists have found 200 different products from the fissi ...
Atomic Structure
... • Electrons closer to the nucleus have the lowest kinetic energy because of attractive forces between the electrons and protons. ...
... • Electrons closer to the nucleus have the lowest kinetic energy because of attractive forces between the electrons and protons. ...
Chapter 4 Review Worksheet. Name
... English chemist and schoolteacher who formulated a theory to describe the structure and chemical reactivity of matter in terms of atoms ...
... English chemist and schoolteacher who formulated a theory to describe the structure and chemical reactivity of matter in terms of atoms ...
Mass Defect - Lamont High
... missing mass is present in the strong nuclear energy holding the nucleus together. Nuclear energy must be very strong because it must overcome the repulsion forces present in the nucleus. This energy holding the nucleus together is called the BINDING ENERGY. ...
... missing mass is present in the strong nuclear energy holding the nucleus together. Nuclear energy must be very strong because it must overcome the repulsion forces present in the nucleus. This energy holding the nucleus together is called the BINDING ENERGY. ...
ppt - Faculty
... • How many protons does each atom of Gold contain? • How many Protons do Uranium atoms contain? • If an Aluminum atom is neutrally charged, how many e- does it contain? ...
... • How many protons does each atom of Gold contain? • How many Protons do Uranium atoms contain? • If an Aluminum atom is neutrally charged, how many e- does it contain? ...
levels of organization and the atom
... unable to cut”. It has two areas: a nucleus and an electron cloud. It contains subatomic particles that are even smaller. ...
... unable to cut”. It has two areas: a nucleus and an electron cloud. It contains subatomic particles that are even smaller. ...
Atom Study Guide
... John Dalton – experiments led to everybody agreeing that there are atoms. Came up with Dalton’s Atomic Theory. All elements are composed of atoms. Atoms are indivisible and indestructible particles. (not true because there are smaller parts within the atom) Atoms of the same element are exactly ...
... John Dalton – experiments led to everybody agreeing that there are atoms. Came up with Dalton’s Atomic Theory. All elements are composed of atoms. Atoms are indivisible and indestructible particles. (not true because there are smaller parts within the atom) Atoms of the same element are exactly ...