Ch.1-Matter and Change
... Elements Types of Elements Metals A metal is an element that is good electrical conductor and a good heat conductor. Properties of Metals most are solid at room temperature malleable - they can be hammered or rolled into thin sheets ductile - they can be drawn into a thin wire conduct electricity a ...
... Elements Types of Elements Metals A metal is an element that is good electrical conductor and a good heat conductor. Properties of Metals most are solid at room temperature malleable - they can be hammered or rolled into thin sheets ductile - they can be drawn into a thin wire conduct electricity a ...
A2 Module 2814: Chains, Rings and Spectroscopy
... EEE 2: Transition elements For the elements up to Ca the 3d orbitals are higher in energy than the 4s orbital. Therefore, after argon (element 18), the 4s orbital is filled: Ca has electron configuration [Ar] 4s2. From scandium on, the 3d orbitals are filled, until they have ten electrons at zinc. T ...
... EEE 2: Transition elements For the elements up to Ca the 3d orbitals are higher in energy than the 4s orbital. Therefore, after argon (element 18), the 4s orbital is filled: Ca has electron configuration [Ar] 4s2. From scandium on, the 3d orbitals are filled, until they have ten electrons at zinc. T ...
Atoms – Building Blocks of Matter Notes
... Aristotle was incorrect but did not have their own theory to submit. At this time chemist did believe, based on experiments, that there were different elements and that an element was a substance that could not be broken down by chemical means. Chemist knew that some substances could transform into ...
... Aristotle was incorrect but did not have their own theory to submit. At this time chemist did believe, based on experiments, that there were different elements and that an element was a substance that could not be broken down by chemical means. Chemist knew that some substances could transform into ...
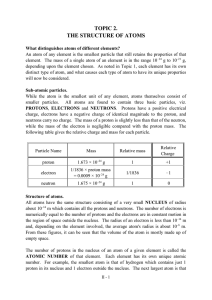
TOPIC 2. THE STRUCTURE OF ATOMS
... than a noble gas atom, and they all behave as does sodium in that relatively little energy is needed to form their +1 cations Li+, Na+, K+, Rb+ and Cs+. Each of these cations has the same electron arrangement as the atom of the noble gas whose atomic number is 1 less than that of the first group ele ...
... than a noble gas atom, and they all behave as does sodium in that relatively little energy is needed to form their +1 cations Li+, Na+, K+, Rb+ and Cs+. Each of these cations has the same electron arrangement as the atom of the noble gas whose atomic number is 1 less than that of the first group ele ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... Can you determine whether or not a solution is active? Can you put metals in order based on their activities? ...
... Can you determine whether or not a solution is active? Can you put metals in order based on their activities? ...
C3 Revision Question Booklet
... were in the order of their relative atomic masses. ......................................................... and .......................................................... ...
... were in the order of their relative atomic masses. ......................................................... and .......................................................... ...
4 ATOMIC STRUCTURE NOTES __ /__ pts
... 4. Which particle controls what element an atom is (hint: See which particle when added changes the element name in the info box)?_________ 5. What do you get when you change the number of neutrons in the nucleus? 6. What 2 particles control the mass of an atom(hint: Look at which particle doesn’t c ...
... 4. Which particle controls what element an atom is (hint: See which particle when added changes the element name in the info box)?_________ 5. What do you get when you change the number of neutrons in the nucleus? 6. What 2 particles control the mass of an atom(hint: Look at which particle doesn’t c ...
TOPIC 2. THE STRUCTURE OF ATOMS
... whose atomic number is 1 less than that of the first group element. Some other elements have atoms which only require one more electron in order to obtain the noble gas arrangement. These atoms are F, Cl, Br and I, all of which are just one electron short of having a filled outer level. In chemical ...
... whose atomic number is 1 less than that of the first group element. Some other elements have atoms which only require one more electron in order to obtain the noble gas arrangement. These atoms are F, Cl, Br and I, all of which are just one electron short of having a filled outer level. In chemical ...
atom
... Radioactive Decay • Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
... Radioactive Decay • Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
CHEM 250Q
... The compounds have different properties because they contain the same elements in different quantities. ...
... The compounds have different properties because they contain the same elements in different quantities. ...
U N I 1. laboratory tools and chemistry techniques.
... • an increase or decrease in the mass of material • a change in the texture of a material 2. Possible answer: The copper would always be present throughout the series of reactions. The copper could be in a different form after each reaction. For example, the copper could become part of a compound, ...
... • an increase or decrease in the mass of material • a change in the texture of a material 2. Possible answer: The copper would always be present throughout the series of reactions. The copper could be in a different form after each reaction. For example, the copper could become part of a compound, ...
2.3 Atomic Mass and Number
... and neutrons, you will find that electrons have an extremely small mass compared to the masses of either protons or neutrons, just like the mass of a penny so extremely small compared to the mass of a bowling ball. On the other hand, the masses of protons and neutrons are fairly similar, with the ma ...
... and neutrons, you will find that electrons have an extremely small mass compared to the masses of either protons or neutrons, just like the mass of a penny so extremely small compared to the mass of a bowling ball. On the other hand, the masses of protons and neutrons are fairly similar, with the ma ...
Chemistry - Volusia County Schools
... Body of Knowledge: the broadest organizational structure used to group content and concepts within the curriculum map Pacing: time frames created by teacher committees, using EOC data, within which the course should be taught in preparation for the Biology EOC Measurement Topics: concepts grouped to ...
... Body of Knowledge: the broadest organizational structure used to group content and concepts within the curriculum map Pacing: time frames created by teacher committees, using EOC data, within which the course should be taught in preparation for the Biology EOC Measurement Topics: concepts grouped to ...
FINAL REVIEW - Normal Community High School Chemistry
... surrounding freely mobile electrons. Most metals contribute more than one mobile electron per atom. Bailar, Jr, Moeller, Kleinberg, Guss, Castellion, Metz, Chemistry, 1984, page 245 ...
... surrounding freely mobile electrons. Most metals contribute more than one mobile electron per atom. Bailar, Jr, Moeller, Kleinberg, Guss, Castellion, Metz, Chemistry, 1984, page 245 ...
Chapter 6 ppt
... • Atoms are so small that light waves are too large to be used to observe them. Scientists use scanning tunneling electron microscopes to provide images of atoms. • However, these images are not an actual picture of the atom. They show an image of the surface of a material at the atomic level. ...
... • Atoms are so small that light waves are too large to be used to observe them. Scientists use scanning tunneling electron microscopes to provide images of atoms. • However, these images are not an actual picture of the atom. They show an image of the surface of a material at the atomic level. ...
Chemistry: Matter and Change
... Radioactive Decay • Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
... Radioactive Decay • Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
Topic_4
... in scientific notation, a mole is 6.02 x 1023 particles. Scientific notation is used to express very small or very large measurements in powers of ten. It expresses quantities by using a number between one and ten, which is then multiplied by ten to a power to give the quantity its proper magnitude. ...
... in scientific notation, a mole is 6.02 x 1023 particles. Scientific notation is used to express very small or very large measurements in powers of ten. It expresses quantities by using a number between one and ten, which is then multiplied by ten to a power to give the quantity its proper magnitude. ...
Chemistry Semester 1 Exam Review Study Island
... everything else about the rabbits' living environment the same, including the amount of water and food provided. After a month, she measures the body fat of the rabbits in both groups. What is the control in Dr. Grey's experiment? A. the group of rabbits that are given the drug B. the group of rabbi ...
... everything else about the rabbits' living environment the same, including the amount of water and food provided. After a month, she measures the body fat of the rabbits in both groups. What is the control in Dr. Grey's experiment? A. the group of rabbits that are given the drug B. the group of rabbi ...
Mendeleev`s Periodic Table
... Figure: The Arrangement of the Elements into Octaves as Proposed by Newlands: The table shown here accompanied a letter from a 27-year-old Newlands to the editor of the journal Chemical News in which he wrote: “If the elements are arranged in the order of their equivalents, with a few slight transp ...
... Figure: The Arrangement of the Elements into Octaves as Proposed by Newlands: The table shown here accompanied a letter from a 27-year-old Newlands to the editor of the journal Chemical News in which he wrote: “If the elements are arranged in the order of their equivalents, with a few slight transp ...
Study Guide for Final #1
... 1.) Know who the important contributors were who helped to derive the different models of the atom. Know what their contributions were. 2.) Be able to describe Dalton’s atomic theory. 3.) Know where the three different subatomic particles are located, their charges, and their relative sizes. 4.) Kno ...
... 1.) Know who the important contributors were who helped to derive the different models of the atom. Know what their contributions were. 2.) Be able to describe Dalton’s atomic theory. 3.) Know where the three different subatomic particles are located, their charges, and their relative sizes. 4.) Kno ...
Chapter 3—Time and Geology
... geochronology (29): The study of time as applied to Earth and planetary history. ...
... geochronology (29): The study of time as applied to Earth and planetary history. ...