Chapter 2 Atoms and Elements
... Modern Atomic Theory and the Laws That Led to It • The theory that all matter is composed of atoms grew out of observations and laws. • The three most important laws that led to the development and acceptance of the atomic theory are as follows: – The law of conservation of mass – The law of defini ...
... Modern Atomic Theory and the Laws That Led to It • The theory that all matter is composed of atoms grew out of observations and laws. • The three most important laws that led to the development and acceptance of the atomic theory are as follows: – The law of conservation of mass – The law of defini ...
atom
... Modern Atomic Theory and the Laws That Led to It • The theory that all matter is composed of atoms grew out of observations and laws. • The three most important laws that led to the development and acceptance of the atomic theory are as follows: – The law of conservation of mass – The law of defini ...
... Modern Atomic Theory and the Laws That Led to It • The theory that all matter is composed of atoms grew out of observations and laws. • The three most important laws that led to the development and acceptance of the atomic theory are as follows: – The law of conservation of mass – The law of defini ...
10/18/11 - Note: Once it is downloaded, click SET
... Group18- Noble gases - full outer shell, unreactive (8 valence electrons) - Ne, Ar, Kr, Xe, Rn ...
... Group18- Noble gases - full outer shell, unreactive (8 valence electrons) - Ne, Ar, Kr, Xe, Rn ...
Elements and the Periodic Table
... (1871−1937) directed highly energetic, positively charged alpha particles at a very thin gold foil. He traced the paths of the alpha particles after they collided with the gold foil. Using mathematical calculations, he showed that the only configuration of the atom that could explain the paths of th ...
... (1871−1937) directed highly energetic, positively charged alpha particles at a very thin gold foil. He traced the paths of the alpha particles after they collided with the gold foil. Using mathematical calculations, he showed that the only configuration of the atom that could explain the paths of th ...
mc_ch03
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
Chapter 3
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
Unit 2.4 Understanding the Elements Listed on the Periodic Table
... Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since 2 + 2 = 4, we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons since the number of electrons must equal the ...
... Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since 2 + 2 = 4, we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons since the number of electrons must equal the ...
09 - Northern Highlands
... sodium, and potassium always combine with oxygen in a ratio of two atoms of metal per atom of oxygen (Figure 9.11). By keeping track of how each element combined with other elements, scientists began to recognize repeating patterns. From this data, they developed the first periodic table of the elem ...
... sodium, and potassium always combine with oxygen in a ratio of two atoms of metal per atom of oxygen (Figure 9.11). By keeping track of how each element combined with other elements, scientists began to recognize repeating patterns. From this data, they developed the first periodic table of the elem ...
Atomic Timeline There are small, negatively charged particles inside
... Atomic Timeline The table below contains a number of statements connected to major discoveries in the development of the atomic theory. ...
... Atomic Timeline The table below contains a number of statements connected to major discoveries in the development of the atomic theory. ...
regents chemistry midterm - irondequoit 2014_entire exam w key
... about periodic trends on your answer sheet. a) Graph the periodic trend in atomic radius for Period 3 on the appropriate graph on your answer sheet. [1pt] b) Explain the cause for the observed periodic trend in atomic radius for Period 3 elements. [1pt] c) Graph the group trend in atomic radius for ...
... about periodic trends on your answer sheet. a) Graph the periodic trend in atomic radius for Period 3 on the appropriate graph on your answer sheet. [1pt] b) Explain the cause for the observed periodic trend in atomic radius for Period 3 elements. [1pt] c) Graph the group trend in atomic radius for ...
Atomic Structure Practice Test
... PTS: 1 DIF: II REF: 1 OBJ: 3 STA: SC.B.1.4.2 26. ANS: The atomic number equals the number of protons in the nucleus of an atom and also equals the number of electrons in the neutral atom. The mass number is the sum of the number of protons and neutrons and can be used, with the atomic number, to fin ...
... PTS: 1 DIF: II REF: 1 OBJ: 3 STA: SC.B.1.4.2 26. ANS: The atomic number equals the number of protons in the nucleus of an atom and also equals the number of electrons in the neutral atom. The mass number is the sum of the number of protons and neutrons and can be used, with the atomic number, to fin ...
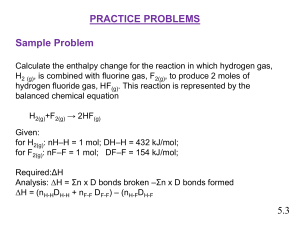
Thermodynamics Practice Problems Presentation
... (1 mol x 432KJ) + (1 mol x 154 KJ) - (2 mol x 565 KJ mol mol mol ∆H = -544 KJ The enthalpy change for the reaction of 1 mol hydrogen gas and 1 mol fluorine gas to ptoduce 2 mol. Hydrogen fluoride is -544 KJ ...
... (1 mol x 432KJ) + (1 mol x 154 KJ) - (2 mol x 565 KJ mol mol mol ∆H = -544 KJ The enthalpy change for the reaction of 1 mol hydrogen gas and 1 mol fluorine gas to ptoduce 2 mol. Hydrogen fluoride is -544 KJ ...
Slide 1
... since there are twice as many oxygen atoms per carbon atom in carbon dioxide than in carbon monoxide, the oxygen mass ratio should be 2 mass of oxygen that combines with 1 g of carbon in carbon dioxide ...
... since there are twice as many oxygen atoms per carbon atom in carbon dioxide than in carbon monoxide, the oxygen mass ratio should be 2 mass of oxygen that combines with 1 g of carbon in carbon dioxide ...
chapter 2 (w)
... Thompson calculated the ratio of the electron’s mass, me, to its electric charge, e. In 1909, U.S. physicist, Robert Millikan had obtained the charge on the electron. (See Figure 2.4) These two discoveries combined provided us with the electron’s mass of 9.109 x 10-31 kg, which is more than 1800 tim ...
... Thompson calculated the ratio of the electron’s mass, me, to its electric charge, e. In 1909, U.S. physicist, Robert Millikan had obtained the charge on the electron. (See Figure 2.4) These two discoveries combined provided us with the electron’s mass of 9.109 x 10-31 kg, which is more than 1800 tim ...
Chemistry 11 – Course Review
... Define: Observation, Interpretation, Qualitative, Quantitative, Data, Experiment, Hypothesis, Theory, Laws, Matter, Chemistry, Physical and Chemical Properties, Malleability, Ductility, Lustre, Viscosity and Diffusion. Review the Phases of Matter. ...
... Define: Observation, Interpretation, Qualitative, Quantitative, Data, Experiment, Hypothesis, Theory, Laws, Matter, Chemistry, Physical and Chemical Properties, Malleability, Ductility, Lustre, Viscosity and Diffusion. Review the Phases of Matter. ...
Chapter 3 - WordPress.com
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
Chapter 5 and 6 Notes
... gain electrons, they become ions. – Cations are positive and are formed by elements on the left side of the periodic chart (metals). – Anions are negative and are formed by elements on the right side of the periodic chart (non-metals). – Ionic charge can be predicted by determining how many electron ...
... gain electrons, they become ions. – Cations are positive and are formed by elements on the left side of the periodic chart (metals). – Anions are negative and are formed by elements on the right side of the periodic chart (non-metals). – Ionic charge can be predicted by determining how many electron ...
Atomic Structure and the Periodic Table
... VERY tiny. An atom is more than one million times smaller than the thickness of a single hair on your head. It would take billions of atoms just to make up the period after the end of this sentence. ...
... VERY tiny. An atom is more than one million times smaller than the thickness of a single hair on your head. It would take billions of atoms just to make up the period after the end of this sentence. ...
Atoms and the Periodic Table Atoms and the Periodic Table
... The regions in an atom where electrons are likely to be found are called orbitals. Within each energy level, electrons occupy orbitals that have the lowest energy. The four different kinds of orbitals are the s, p, d, and f orbitals. The simplest kind of orbital is an s orbital. An s orbital can hav ...
... The regions in an atom where electrons are likely to be found are called orbitals. Within each energy level, electrons occupy orbitals that have the lowest energy. The four different kinds of orbitals are the s, p, d, and f orbitals. The simplest kind of orbital is an s orbital. An s orbital can hav ...
Worlds Within Worlds. The Story of Nuclear Energy Vol I
... atom. If he set the mass of the hydrogen atom at 1 (just for convenience) then the mass of the oxygen atom ought to be set at 8. These comparative, or relative, numbers were said to be “atomic weights”, so that what Dalton was suggesting was that the atomic weight of hydrogen ...
... atom. If he set the mass of the hydrogen atom at 1 (just for convenience) then the mass of the oxygen atom ought to be set at 8. These comparative, or relative, numbers were said to be “atomic weights”, so that what Dalton was suggesting was that the atomic weight of hydrogen ...
Spectroscopy In Oceanography
... neither element, but none concentrates both elements. Ti may be concentrated by some ascidians over one million times (4). Nicholls eta/ (5). employing emission spectrographic techniques for analysis of plankton (small, often microscopic plants and animals that live at or near the sea surface and wh ...
... neither element, but none concentrates both elements. Ti may be concentrated by some ascidians over one million times (4). Nicholls eta/ (5). employing emission spectrographic techniques for analysis of plankton (small, often microscopic plants and animals that live at or near the sea surface and wh ...
atoms - Moodle
... that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not have the same relative number Atoms, Molecules, of atoms. and Ions © 2015 ...
... that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not have the same relative number Atoms, Molecules, of atoms. and Ions © 2015 ...
Stoichiometry
... hydrogen atoms in 600g of propane, C3 H8 C = 12 , H = 1. MW = ( 3 x 12 ) + ( 8 x 1 ) = 44 amu Number of moles of propane = 600 g x 1 mol = 13.63 mol 44 g 1 mole of C3H8 contains 3 mol of C atoms and contains 8 mol of H atoms. It contains also Avogadro's number of C3 H8 molecules. ...
... hydrogen atoms in 600g of propane, C3 H8 C = 12 , H = 1. MW = ( 3 x 12 ) + ( 8 x 1 ) = 44 amu Number of moles of propane = 600 g x 1 mol = 13.63 mol 44 g 1 mole of C3H8 contains 3 mol of C atoms and contains 8 mol of H atoms. It contains also Avogadro's number of C3 H8 molecules. ...
Science SOL CH
... Part Two – Determining the Average Mass for Different Samples of Beanium 1. Place TWO small plastic cups (one marked “A” and the other marked “B”) on the electronic scale and press the zero button. The scale should read “0.00 g” with the two cups on the pan. 2. Place 2 atoms of Isotope A into the A ...
... Part Two – Determining the Average Mass for Different Samples of Beanium 1. Place TWO small plastic cups (one marked “A” and the other marked “B”) on the electronic scale and press the zero button. The scale should read “0.00 g” with the two cups on the pan. 2. Place 2 atoms of Isotope A into the A ...