Glossary: Chemical bonds
... The atomic weight given on the periodic table is a weighted average of isotopic masses found in a typical terrestrial sample of the element. Atom. Compare with molecule and ion. An atom is the smallest particle of an element that retains the chemical properties of the element. Atoms are electrically ...
... The atomic weight given on the periodic table is a weighted average of isotopic masses found in a typical terrestrial sample of the element. Atom. Compare with molecule and ion. An atom is the smallest particle of an element that retains the chemical properties of the element. Atoms are electrically ...
2(g)
... Excess and limiting reagents refer to the reactant that will run out first and stop more product from forming. ...
... Excess and limiting reagents refer to the reactant that will run out first and stop more product from forming. ...
Atoms, Elements, Compounds, and Mixtures
... Thomson’s experiments. However, the answers inspired new questions. If atoms contain one or more negatively charged particles, then all matter, which is made of atoms, should be negatively charged as well. But all matter isn’t negatively charged. Could it be that atoms also contain some positive cha ...
... Thomson’s experiments. However, the answers inspired new questions. If atoms contain one or more negatively charged particles, then all matter, which is made of atoms, should be negatively charged as well. But all matter isn’t negatively charged. Could it be that atoms also contain some positive cha ...
0 Review Presentations
... • The IS (International System of units) is used all around the world. Except America. • It was created in 1799 and was used temporarily to replace the previously existing system and then replaced it for good since it as easier to understand. ...
... • The IS (International System of units) is used all around the world. Except America. • It was created in 1799 and was used temporarily to replace the previously existing system and then replaced it for good since it as easier to understand. ...
© NCERT not to be republished
... 45. When orange solution containing Cr2O72– ion is treated with an alkali, a yellow solution is formed and when H+ ions are added to yellow solution, an orange solution is obtained. Explain why does this happen? 46. A solution of KMnO4 on reduction yields either a colourless solution or a brown prec ...
... 45. When orange solution containing Cr2O72– ion is treated with an alkali, a yellow solution is formed and when H+ ions are added to yellow solution, an orange solution is obtained. Explain why does this happen? 46. A solution of KMnO4 on reduction yields either a colourless solution or a brown prec ...
Counting Atoms
... exam scores and 40% on laboratory explorations. • Your exam scores would count more heavily toward your final grade. • In this section, you will learn that the atomic mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element. ...
... exam scores and 40% on laboratory explorations. • Your exam scores would count more heavily toward your final grade. • In this section, you will learn that the atomic mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element. ...
sec 3- Counting atoms - Nutley Public Schools
... exam scores and 40% on laboratory explorations. • Your exam scores would count more heavily toward your final grade. • In this section, you will learn that the atomic mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element. ...
... exam scores and 40% on laboratory explorations. • Your exam scores would count more heavily toward your final grade. • In this section, you will learn that the atomic mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element. ...
sec 3- Counting atoms - Nutley Public Schools
... exam scores and 40% on laboratory explorations. • Your exam scores would count more heavily toward your final grade. • In this section, you will learn that the atomic mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element. ...
... exam scores and 40% on laboratory explorations. • Your exam scores would count more heavily toward your final grade. • In this section, you will learn that the atomic mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element. ...
Part V Elements And Atomic Weights
... To put it simply, elements are the basic building blocks of the chemical and physical world, as we know it. While many of us remember this basic concept from high school chemistry class, details such as the name, abbreviation, and atomic weight2 of each element are probably a bit fuzzy. This is unde ...
... To put it simply, elements are the basic building blocks of the chemical and physical world, as we know it. While many of us remember this basic concept from high school chemistry class, details such as the name, abbreviation, and atomic weight2 of each element are probably a bit fuzzy. This is unde ...
InorgCh8.2
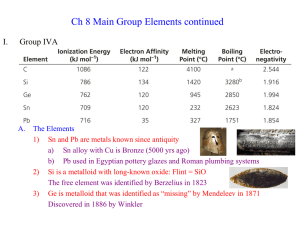
... c) Br2 in 1826 by Balard; red-brown liquid that easily vaporizes d) F2 in 1886 by Moissan; so reactive it is hard to even handle e) At2 in 1940 during particle collisions (radioactive with ½ life = only 8 hours) 3) Chemistry dominated by ready reduction to Xa) Excellent oxidizing agents (is reduced ...
... c) Br2 in 1826 by Balard; red-brown liquid that easily vaporizes d) F2 in 1886 by Moissan; so reactive it is hard to even handle e) At2 in 1940 during particle collisions (radioactive with ½ life = only 8 hours) 3) Chemistry dominated by ready reduction to Xa) Excellent oxidizing agents (is reduced ...
TOPIC 2. THE STRUCTURE OF ATOMS
... whose atomic number is 1 less than that of the first group element. Some other elements have atoms which only require one more electron in order to obtain the noble gas arrangement. These atoms are F, Cl, Br and I, all of which are just one electron short of having a filled outer level. In chemical ...
... whose atomic number is 1 less than that of the first group element. Some other elements have atoms which only require one more electron in order to obtain the noble gas arrangement. These atoms are F, Cl, Br and I, all of which are just one electron short of having a filled outer level. In chemical ...
Atoms – Building Blocks of Matter Notes
... This idea was widely supported and accepted until the late 1700’s and he too had NO experimental evidence to support his idea. ...
... This idea was widely supported and accepted until the late 1700’s and he too had NO experimental evidence to support his idea. ...
File
... The properties of mass and volume can be used to describe another important general property of matter called density. Density is the mass per unit volume of an object. Density is important property because it allows you to compare different types of matter. Suppose you were asked to determine wheth ...
... The properties of mass and volume can be used to describe another important general property of matter called density. Density is the mass per unit volume of an object. Density is important property because it allows you to compare different types of matter. Suppose you were asked to determine wheth ...
atom
... 3- Atoms of different elements can combine in simple whole number ratios to form compounds. 4 – Chemical reactions occur when atoms are separated, joined or rearranged. However, atoms of one element are not changed into atoms of another element by a chemical reaction. NC Competency Goal 2 ...
... 3- Atoms of different elements can combine in simple whole number ratios to form compounds. 4 – Chemical reactions occur when atoms are separated, joined or rearranged. However, atoms of one element are not changed into atoms of another element by a chemical reaction. NC Competency Goal 2 ...
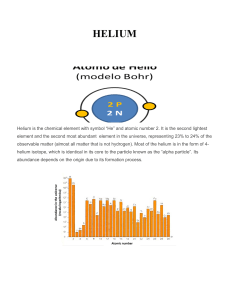
HELIUM - IDC
... observable matter (almost all matter that is not hydrogen). Most of the helium is in the form of 4helium isotope, which is identical in its core to the particle known as the “alpha particle”. Its abundance depends on the origin due to its formation process. ...
... observable matter (almost all matter that is not hydrogen). Most of the helium is in the form of 4helium isotope, which is identical in its core to the particle known as the “alpha particle”. Its abundance depends on the origin due to its formation process. ...
Chapter 4 “Atomic Structure”
... • Dalton studied the ratios in which elements combined during chemical reactions (his experiments showed that the ratios in which elements combined were whole numbers) • Based on the data from his experiments, he then formulated additional hypotheses and theories to explain his observations and test ...
... • Dalton studied the ratios in which elements combined during chemical reactions (his experiments showed that the ratios in which elements combined were whole numbers) • Based on the data from his experiments, he then formulated additional hypotheses and theories to explain his observations and test ...
1994–PTAS, Inc - mvhs
... 1. If two atomic species are isotopes, then (A) both atoms must have identical nuclei. (B) the nuclei of both atoms contain the same number of neutrons. (C) the nuclei of both atoms contain the same number of protons. (D) both atoms must have the same mass. 2. What is the maximum number of electrons ...
... 1. If two atomic species are isotopes, then (A) both atoms must have identical nuclei. (B) the nuclei of both atoms contain the same number of neutrons. (C) the nuclei of both atoms contain the same number of protons. (D) both atoms must have the same mass. 2. What is the maximum number of electrons ...
atom - WordPress.com
... Composition of the Atomic Nucleus, continued Forces in the Nucleus • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The sho ...
... Composition of the Atomic Nucleus, continued Forces in the Nucleus • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The sho ...
Course Map_2011-2012 - Kenwood Academy High School
... 12.11.74 Understand that the magnitude of a force F is defined as F = ma (Force equals Mass times Acceleration). Know how to perform such calculations. Understand that whenever one object exerts force on another, a force equal in magnitude and opposite in direction is exerted on the first object. Un ...
... 12.11.74 Understand that the magnitude of a force F is defined as F = ma (Force equals Mass times Acceleration). Know how to perform such calculations. Understand that whenever one object exerts force on another, a force equal in magnitude and opposite in direction is exerted on the first object. Un ...
Practice Qs - Unit 6a
... 3. Below are a list of formulas. Write the empirical formula (if not already empirical). ...
... 3. Below are a list of formulas. Write the empirical formula (if not already empirical). ...
notes - unit 2 - atomic theory_key_2012
... ALL ELECTRONS MUST be drawn BOHRing 1. Look up electron configuration of element at hand on Periodic Table. If you are working with an ion, add/subtract the proper amount of electrons the last or VALENCE number in the configuration. Example: Oxygen is 2-6 2. Draw a square for the nucleus and notat ...
... ALL ELECTRONS MUST be drawn BOHRing 1. Look up electron configuration of element at hand on Periodic Table. If you are working with an ion, add/subtract the proper amount of electrons the last or VALENCE number in the configuration. Example: Oxygen is 2-6 2. Draw a square for the nucleus and notat ...
Chapter 2 "Elements, Atoms, and the Periodic Table"
... The hardest material in the human body is tooth enamel. It has to be hard so that our teeth can serve us for a lifetime of biting and chewing; however, tough as it is, tooth enamel is susceptible to chemical attack. Acids found in some foods or made by bacteria that feed on food residues on our teet ...
... The hardest material in the human body is tooth enamel. It has to be hard so that our teeth can serve us for a lifetime of biting and chewing; however, tough as it is, tooth enamel is susceptible to chemical attack. Acids found in some foods or made by bacteria that feed on food residues on our teet ...
Atom 3 Isotopes - Solon City Schools
... Atoms with the same number of protons & electrons but a different number of neutrons. They are the same element, are chemically identical and undergo the exact same chemical reactions They have different masses (different mass number). All isotopes are used to calculate average atomic mass (this mas ...
... Atoms with the same number of protons & electrons but a different number of neutrons. They are the same element, are chemically identical and undergo the exact same chemical reactions They have different masses (different mass number). All isotopes are used to calculate average atomic mass (this mas ...