Final Exam review semester 1
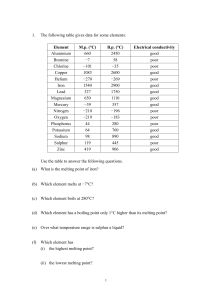
... 1. Ninety-nine percent of all the matter that can be observed in the universe exists as 2. Hydrochloric acid, HCl, is added to solid NaOH. After the reaction is complete, NaCl dissolved in water remains. What are the products of this chemical reaction? ...
... 1. Ninety-nine percent of all the matter that can be observed in the universe exists as 2. Hydrochloric acid, HCl, is added to solid NaOH. After the reaction is complete, NaCl dissolved in water remains. What are the products of this chemical reaction? ...
Answers
... (c) Lithium would float on water, [1] producing gas steadily. [1] (d) Potassium would melt to a silvery ball [1] which moves about very quickly on the water surface, [1] producing a hissing sound, [1] burning spontaneously with a lilac flame [1] before finally disappearing completely. [1] (e) It wou ...
... (c) Lithium would float on water, [1] producing gas steadily. [1] (d) Potassium would melt to a silvery ball [1] which moves about very quickly on the water surface, [1] producing a hissing sound, [1] burning spontaneously with a lilac flame [1] before finally disappearing completely. [1] (e) It wou ...
physical earth science
... 2. John Dalton developed an atomic theory a. 1808 English schoolteacher – proposed his theory b. Proposed that atoms could not be divided (today we know differently) c. Proposed all atoms of one element were the same d. Proposed different atoms could join to form compounds e. Considered the foundati ...
... 2. John Dalton developed an atomic theory a. 1808 English schoolteacher – proposed his theory b. Proposed that atoms could not be divided (today we know differently) c. Proposed all atoms of one element were the same d. Proposed different atoms could join to form compounds e. Considered the foundati ...
Atomic Theory
... was continuous-did not believe in atoms • His opinion was accepted for nearly 2000 years ...
... was continuous-did not believe in atoms • His opinion was accepted for nearly 2000 years ...
Matter
... • Element • Physical – Collection of the combination same type of atom elements and/or – Cannot be compounds or decomposed both. • Compound • USUALLY – 2 or more different heterogeneous atoms chemically bonded together. ...
... • Element • Physical – Collection of the combination same type of atom elements and/or – Cannot be compounds or decomposed both. • Compound • USUALLY – 2 or more different heterogeneous atoms chemically bonded together. ...
Elements, Compounds, Mixtures
... • Horace Deming, 1923: the popular periodic table layout, also known as the common or standard form. ...
... • Horace Deming, 1923: the popular periodic table layout, also known as the common or standard form. ...
CS 211 – Spring 2017 Lab 4: Molar Mass
... Open this spreadsheet and copy/paste the entire table to Sheet2 of your lab. Note that on row 3 the element name is a single blank (“ “), and zeros are recorded for the numbers. This “element” is added so that BLANK can be selected on the drop-down box later. - Rename Sheet2 to Elements and Sheet 1 ...
... Open this spreadsheet and copy/paste the entire table to Sheet2 of your lab. Note that on row 3 the element name is a single blank (“ “), and zeros are recorded for the numbers. This “element” is added so that BLANK can be selected on the drop-down box later. - Rename Sheet2 to Elements and Sheet 1 ...
Intensive Chemistry: the Structure of Matter
... other. Watch what happens when a magnet comes near the tube. Which side of the magnet do you think they used? Source: http://chem.illinois.edu/CLCwebsite/demos.html ...
... other. Watch what happens when a magnet comes near the tube. Which side of the magnet do you think they used? Source: http://chem.illinois.edu/CLCwebsite/demos.html ...
Chapter 4: The Structure of the Atom &
... o Above the band of stability – too many _____________; Below the band of stability – too many _______________ or too few ______________ o BETA DECAY: For elements above the band of stability (too many neutrons) A NEUTRON will decay into a PROTON (stays in the nucleus) and an ELECTRON (leaves the ...
... o Above the band of stability – too many _____________; Below the band of stability – too many _______________ or too few ______________ o BETA DECAY: For elements above the band of stability (too many neutrons) A NEUTRON will decay into a PROTON (stays in the nucleus) and an ELECTRON (leaves the ...
File
... copper were known at the time, the Egyptians had no idea that these metals, in fact, elements. Metallurgy – the science behind the knowledge that medals could be obtained from ore and purified became an important part of the culture of Egypt and Babylon. A close association between priests, temples ...
... copper were known at the time, the Egyptians had no idea that these metals, in fact, elements. Metallurgy – the science behind the knowledge that medals could be obtained from ore and purified became an important part of the culture of Egypt and Babylon. A close association between priests, temples ...
UNIT_6___ELECTRON___CONFIGURATIONS__NOTES
... - Note: One exception to this rule : The 1st shell can only hold 2eFAMILIES OF ELEMENTS: Vertical Columns on the periodic table Elements with similar chemical properties are called chemical families ( because they have the same # e- in the outer shell) PERIODS OF ELEMENTS: Horizontal rows with ele ...
... - Note: One exception to this rule : The 1st shell can only hold 2eFAMILIES OF ELEMENTS: Vertical Columns on the periodic table Elements with similar chemical properties are called chemical families ( because they have the same # e- in the outer shell) PERIODS OF ELEMENTS: Horizontal rows with ele ...
Elements
... ❖ It is a pure substance that cannot be separated into simpler substances by physical or chemical means. ...
... ❖ It is a pure substance that cannot be separated into simpler substances by physical or chemical means. ...
Lecture 2
... • An element is defined its atomic number. Changing the number of protons in an atom (as in a nuclear reaction) changes the element. • While atoms of the same element must have the same atomic number, they may have different mass numbers. If so, they are referred to as isotopes. Most elements have m ...
... • An element is defined its atomic number. Changing the number of protons in an atom (as in a nuclear reaction) changes the element. • While atoms of the same element must have the same atomic number, they may have different mass numbers. If so, they are referred to as isotopes. Most elements have m ...
Structure of the Atom
... • The mass number is the total number of protons and neutrons in the nucleus. • Atoms can have different numbers of neutrons. • Atoms that have different numbers of neutrons are called isotopes. ...
... • The mass number is the total number of protons and neutrons in the nucleus. • Atoms can have different numbers of neutrons. • Atoms that have different numbers of neutrons are called isotopes. ...
Periodic Trends
... atoms. Let’s take Na and Cl for example. They are both on n=3. But Cl has 17 protons pulling on n=3 and Na only has 11 protons pulling on n=3. Cl is smaller because it’s nuclear charge is greater. That fact is very important for the trends. Once we can see that the nonmetals tend to be smaller with ...
... atoms. Let’s take Na and Cl for example. They are both on n=3. But Cl has 17 protons pulling on n=3 and Na only has 11 protons pulling on n=3. Cl is smaller because it’s nuclear charge is greater. That fact is very important for the trends. Once we can see that the nonmetals tend to be smaller with ...
Protons, electrons and neutrons worksheet
... Atomic symbol is the symbol you find for each element shown in the periodic table. Magnesium symbol is Mg Gold symbol is Au Potassium symbol is K Phosphorous symbol is P Note: First letter of the element is not always the symbol. Atomic number is the number on the top left of atomic symbol in period ...
... Atomic symbol is the symbol you find for each element shown in the periodic table. Magnesium symbol is Mg Gold symbol is Au Potassium symbol is K Phosphorous symbol is P Note: First letter of the element is not always the symbol. Atomic number is the number on the top left of atomic symbol in period ...
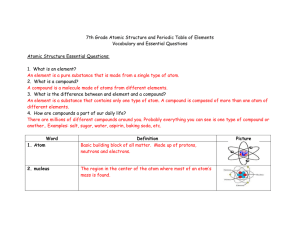
7th Grade Atomic Structure and Periodic Table of Elements
... A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is composed of more than one atom of different elements. 4. How are compounds a part of our daily li ...
... A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is composed of more than one atom of different elements. 4. How are compounds a part of our daily li ...
CH 5 Periodic Law
... - term comes from noble people, did not associate with anyone other then their kind - characterized by an octet of electrons in the outermost energy level; (happy) - exception of helium - very stable, (unreactive) - colorless, odorless - practical applications: balloons, illumination ...
... - term comes from noble people, did not associate with anyone other then their kind - characterized by an octet of electrons in the outermost energy level; (happy) - exception of helium - very stable, (unreactive) - colorless, odorless - practical applications: balloons, illumination ...
3-ELEMENTS AND THE ATOMIC MODEL. C4.8A Identify the
... C4.8A Identify the location, relative mass, and charge for electrons, protons, and neutrons. C4.8B Describe the atom as mostly empty space with an extremely small, dense nucleus consisting of the protons and neutrons and an electron cloud surrounding the nucleus. C4.8C Recognize that protons repel e ...
... C4.8A Identify the location, relative mass, and charge for electrons, protons, and neutrons. C4.8B Describe the atom as mostly empty space with an extremely small, dense nucleus consisting of the protons and neutrons and an electron cloud surrounding the nucleus. C4.8C Recognize that protons repel e ...
introductory chemistry
... Elements are substances that contain only one type of atom. Hydrogen gas is an element as it contains only hydrogen atoms. Compounds contain the atoms of two or more different elements joined together. Water is a compound that consists of hydrogen and oxygen atoms joined together. There are nearly 1 ...
... Elements are substances that contain only one type of atom. Hydrogen gas is an element as it contains only hydrogen atoms. Compounds contain the atoms of two or more different elements joined together. Water is a compound that consists of hydrogen and oxygen atoms joined together. There are nearly 1 ...
The Chemical Context of Life
... and protons have a mass of 1 dalton (same as atomic mass unit) Atomic number – number of protons ...
... and protons have a mass of 1 dalton (same as atomic mass unit) Atomic number – number of protons ...
Compound vs Element chart
... • consists of only one kind of atom, • cannot be broken down into a simpler type of matter by either physical or chemical means, and • can exist as either atoms (e.g. argon) or molecules (e.g., nitrogen). A molecule consists of two or more atoms of the same element, or different elements, that are c ...
... • consists of only one kind of atom, • cannot be broken down into a simpler type of matter by either physical or chemical means, and • can exist as either atoms (e.g. argon) or molecules (e.g., nitrogen). A molecule consists of two or more atoms of the same element, or different elements, that are c ...
Chapter 5 “Atomic Structure and the Periodic table”
... 2)Atoms of the same element are identical. Atoms of any one element are different from those of any other element. 3)Atoms of different elements combine in simple whole-number ratios to form chemical compounds 4)In chemical reactions, atoms are combined, separated, or rearranged – but never changed ...
... 2)Atoms of the same element are identical. Atoms of any one element are different from those of any other element. 3)Atoms of different elements combine in simple whole-number ratios to form chemical compounds 4)In chemical reactions, atoms are combined, separated, or rearranged – but never changed ...