Chemistry lecture notes
... different number of neutrons). Isotopes behave the same chemically, because they are the same element. The only difference is that one is heavier than the other, because of the additional neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon. Carbon-12 has 6 neutrons; carbon ...
... different number of neutrons). Isotopes behave the same chemically, because they are the same element. The only difference is that one is heavier than the other, because of the additional neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon. Carbon-12 has 6 neutrons; carbon ...
The Atom - Mrs. Ellis` Science Class!
... ____________ the ________________ in specific and ______________ paths o However, an electron’s _____________ location _________________ be determined o Electrons exist in energy levels called ________________________ o The number of ____________ orbitals depends on how many _________________ an ato ...
... ____________ the ________________ in specific and ______________ paths o However, an electron’s _____________ location _________________ be determined o Electrons exist in energy levels called ________________________ o The number of ____________ orbitals depends on how many _________________ an ato ...
How Atoms Differ Elements, Isotopes, and Ions
... Atoms are arranged in order by their atomic #. ...
... Atoms are arranged in order by their atomic #. ...
04-Atoms_ molecules_ ions_etc
... same number of protons, but different number of neutrons •Z constant, A variable ...
... same number of protons, but different number of neutrons •Z constant, A variable ...
Lesson Outline - WordPress.com
... * The atoms of one element Cannot be converted into atoms of another element in a chemical reaction. * All atoms of one element have the same properties, such has mass and size * Atoms of different elements combine in specific proportions to form compounds ...
... * The atoms of one element Cannot be converted into atoms of another element in a chemical reaction. * All atoms of one element have the same properties, such has mass and size * Atoms of different elements combine in specific proportions to form compounds ...
bonding notes for votech
... Find the oxidation number for each element and write it above each element without the sign. Swap the oxidation numbers and write the formula. ...
... Find the oxidation number for each element and write it above each element without the sign. Swap the oxidation numbers and write the formula. ...
Activity 17 Follow-up
... neutrons, but always has the same number of protons •The atomic weight is the average weight of all the known isotopes of the element •The element which appears on the periodic table is the isotope which is most abundant ...
... neutrons, but always has the same number of protons •The atomic weight is the average weight of all the known isotopes of the element •The element which appears on the periodic table is the isotope which is most abundant ...
AP Unit 0: Chemical Foundations
... ◦ Compounds are formed by the combination of different atoms in the ratio of small whole numbers. ◦ A chemical reaction involves only the rearrangement of atoms; ◦ atoms are not created / destroyed ...
... ◦ Compounds are formed by the combination of different atoms in the ratio of small whole numbers. ◦ A chemical reaction involves only the rearrangement of atoms; ◦ atoms are not created / destroyed ...
Chemistry Study Guide
... Periodic Table The first version of the modern periodic table was created by Dmitri Mendeleev. He was Russian chemist that classified matter based on physical and chemical properties. He organized the known elements of the time by increasing atomic mass. He left gaps in his table where he believed n ...
... Periodic Table The first version of the modern periodic table was created by Dmitri Mendeleev. He was Russian chemist that classified matter based on physical and chemical properties. He organized the known elements of the time by increasing atomic mass. He left gaps in his table where he believed n ...
S1-2-02: What is the basic subatomic structure of an atom?
... A pure substance that can be only be broken down by chemical means ...
... A pure substance that can be only be broken down by chemical means ...
Nuclear Chemistry PowerPoint
... parts, releasing a large amount of energy in the process. Most commonly this is done by "firing" a neutron at the nucleus of an atom. The energy of the neutron "bullet" causes the target element to split into two (or more) elements that are lighter than the parent atom. • During the fission of U235, ...
... parts, releasing a large amount of energy in the process. Most commonly this is done by "firing" a neutron at the nucleus of an atom. The energy of the neutron "bullet" causes the target element to split into two (or more) elements that are lighter than the parent atom. • During the fission of U235, ...
Name: Date: Period: Who is the Father of Atomic Theory? What
... 7. Radon-226 has a half life of 1600 years. If we start with 2000 g of radon, how much is left after 4800 years? 8. What type of radioactive reaction occurs when a large nucleus breaks into fragments and gives off radiation? 9. What type of radioactive reaction occurs when two light nuclei collide ...
... 7. Radon-226 has a half life of 1600 years. If we start with 2000 g of radon, how much is left after 4800 years? 8. What type of radioactive reaction occurs when a large nucleus breaks into fragments and gives off radiation? 9. What type of radioactive reaction occurs when two light nuclei collide ...
22-Introduction to Radioactivity
... chemical element and, when this happens, emit (give off) energy. Today, people make use of radioactive isotopes in many ways. Some are beneficial (good), such as medical treatment and creating electrical energy. Others are harmful and dangerous, such as atomic bombs. In this activity, you will first ...
... chemical element and, when this happens, emit (give off) energy. Today, people make use of radioactive isotopes in many ways. Some are beneficial (good), such as medical treatment and creating electrical energy. Others are harmful and dangerous, such as atomic bombs. In this activity, you will first ...
Slide 1 - Effingham County Schools
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
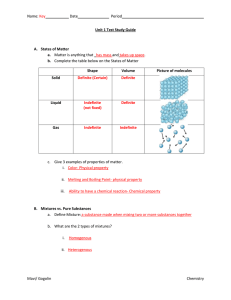
Exam Review/SLO 1 Topics Mixtures Have two or more different
... Depend on type of matter present (density, melting point, boiling point, etc.) Do not depend on how much matter is present Physical Properties Properties which can be observed without a change in identity (chemical make-up) Examples: boiling point, melting point, density Density = mass / volume (alt ...
... Depend on type of matter present (density, melting point, boiling point, etc.) Do not depend on how much matter is present Physical Properties Properties which can be observed without a change in identity (chemical make-up) Examples: boiling point, melting point, density Density = mass / volume (alt ...
UNIT 5 REVIEW PROBLEMS
... 14. It can be predicted that element 118 will have properties similar to ___. a. b. c. d. ...
... 14. It can be predicted that element 118 will have properties similar to ___. a. b. c. d. ...
chapter_17_atomic_structure_review
... passed through.(like charges repel) • Rutherford discovered the nucleus as a result of his experiment ...
... passed through.(like charges repel) • Rutherford discovered the nucleus as a result of his experiment ...
Study Guide Answer Key
... by which J.J. Thomson demonstrated that cathode rays could be deflected by a magnetic field, and that their negative charge was not a separate phenomenon. i. ...
... by which J.J. Thomson demonstrated that cathode rays could be deflected by a magnetic field, and that their negative charge was not a separate phenomenon. i. ...
The Atom Power point - Effingham County Schools
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
... • Aristotle was wrong. However, his theory persisted for 2000 years. ...
nuclear chemistry - La Salle High School
... A. The nucleus of an atom is stable if it does NOT change into another nuclide without adding outside energy. B. Look at each element, determine what nuclides of the elements are stable, plot stable nuclides on graph. No. of protons x axis No. of neutrons y-axis ...
... A. The nucleus of an atom is stable if it does NOT change into another nuclide without adding outside energy. B. Look at each element, determine what nuclides of the elements are stable, plot stable nuclides on graph. No. of protons x axis No. of neutrons y-axis ...
Final Exam Class Review - Mrs. Kittrell`s Science Classes
... • Make a given table that lists the information you are given. BE SURE to include the item you are to find! • USE the Reference sheet! Find the equation that fits what you have. • Put the item you need to find on one side of the equals sign. • Add the other numbers and punch in the ...
... • Make a given table that lists the information you are given. BE SURE to include the item you are to find! • USE the Reference sheet! Find the equation that fits what you have. • Put the item you need to find on one side of the equals sign. • Add the other numbers and punch in the ...