Biochemistry I (CHE 418 / 5418)
... Answers to odd numbered problems in textbook are found in the book’s index. ...
... Answers to odd numbered problems in textbook are found in the book’s index. ...
File
... Protons (+) and neutrons (neutral) The nucleus makes up 99.9% of the mass of the atom The electrons are present in a cloud surrounding the ...
... Protons (+) and neutrons (neutral) The nucleus makes up 99.9% of the mass of the atom The electrons are present in a cloud surrounding the ...
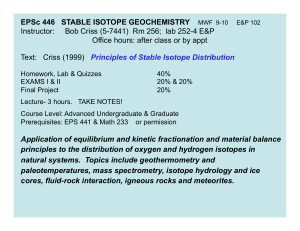
EPSc 446 STABLE ISOTOPE GEOCHEMISTRY Instructor: Bob Criss
... Primordial composition; stellar fuel! ...
... Primordial composition; stellar fuel! ...
The Basis of All Materials
... Same Z, different N Carbon (C ) Z = 6 Carbon-12 Carbon-13 Carbon-14 ...
... Same Z, different N Carbon (C ) Z = 6 Carbon-12 Carbon-13 Carbon-14 ...
Biochemistry Introduction day 1
... Chemical Reactions: when elements and compounds interact with each other to form new substances. Reactant: A substance that undergoes a chemical reaction. ...
... Chemical Reactions: when elements and compounds interact with each other to form new substances. Reactant: A substance that undergoes a chemical reaction. ...
Example of calculating average atomic mass
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
Models Atoms - Hardy Science
... If the statement is true, write true. If the statement is false, change the underlined word or words to make the statement true. 5. ____________________ An element’s mass number tells the number of protons in its nucleus. 6. ____________________ Negatively charged particles in an atom are called ele ...
... If the statement is true, write true. If the statement is false, change the underlined word or words to make the statement true. 5. ____________________ An element’s mass number tells the number of protons in its nucleus. 6. ____________________ Negatively charged particles in an atom are called ele ...
Dating the Earth Power Point
... “radioactive” atoms, each with a different half-life. Half-life is a common way to describe the length of time it takes for half the atoms in a particular element to decay. ...
... “radioactive” atoms, each with a different half-life. Half-life is a common way to describe the length of time it takes for half the atoms in a particular element to decay. ...
Chemistry FINAL: CONTENT Review Packet
... 14. Suppose an unknown radioactive substance with a mass of 120 g has a half-life of 6 months. How much of the substance will remain after 2 years? 7.5g ...
... 14. Suppose an unknown radioactive substance with a mass of 120 g has a half-life of 6 months. How much of the substance will remain after 2 years? 7.5g ...
Welcome to my class - Doral Academy Preparatory
... fundamental particles that make up matter. Yet all matter is composed of such particles, which are called atoms. An atom is the smallest particle of an element that retains its identity in a chemical reaction” ...
... fundamental particles that make up matter. Yet all matter is composed of such particles, which are called atoms. An atom is the smallest particle of an element that retains its identity in a chemical reaction” ...
Course Syllabus - Honors Chemistry
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. a. Atomic number and atomic mass. b. Identify metals, semimetals, nonmetals, halogens, alkali metals, alkaline earth ...
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. a. Atomic number and atomic mass. b. Identify metals, semimetals, nonmetals, halogens, alkali metals, alkaline earth ...
Intro to Chapter 5 Development of the Periodic Table
... What properties of atoms is responsible for the periodic variations? To understand how, it s necessary to look first at the nature of visible line and other forms of radiant energy. Historically, studies of the interaction of radiant energy with matter provided immense insight into the atomic struct ...
... What properties of atoms is responsible for the periodic variations? To understand how, it s necessary to look first at the nature of visible line and other forms of radiant energy. Historically, studies of the interaction of radiant energy with matter provided immense insight into the atomic struct ...
Slide 1
... - a given compound always contains the same elements in the same proportions by mass ...
... - a given compound always contains the same elements in the same proportions by mass ...
review-basics-atomic-structure-and-electron-configurations-v1
... c.) The particle that can occur in different numbers in atoms of the same neutral element ______ d.) Held in energy levels around the nucleus. ______ e.) The negatively-charged particle. ______ f.) The particle with the negligible mass. ______ g.) The number of these particles is found by subtractin ...
... c.) The particle that can occur in different numbers in atoms of the same neutral element ______ d.) Held in energy levels around the nucleus. ______ e.) The negatively-charged particle. ______ f.) The particle with the negligible mass. ______ g.) The number of these particles is found by subtractin ...
Atoms and the Periodic Table
... ◦ The nuclei of the atoms are continually decaying to produce different elements. ◦ Elements with atomic number greater than 92 are man-made, and from 84 and greater are radioactive. ...
... ◦ The nuclei of the atoms are continually decaying to produce different elements. ◦ Elements with atomic number greater than 92 are man-made, and from 84 and greater are radioactive. ...
The Atom
... An atom has three parts: Proton = positive Neutron = no charge Electron = negative The proton & neutron are found in the center of the atom, a place called the nucleus. The electrons orbit the nucleus. ...
... An atom has three parts: Proton = positive Neutron = no charge Electron = negative The proton & neutron are found in the center of the atom, a place called the nucleus. The electrons orbit the nucleus. ...
PowerPoint プレゼンテーション
... How do I calculate the average atomic mass of an element? Look at just about any element on the periodic table. Do you notice that most of the atomic masses listed are not whole numbers. ...
... How do I calculate the average atomic mass of an element? Look at just about any element on the periodic table. Do you notice that most of the atomic masses listed are not whole numbers. ...
Unit 2 – Atomic Theory
... Element Symbol with mass number and atomic number Can also be the element name dash mass number Mass Number ...
... Element Symbol with mass number and atomic number Can also be the element name dash mass number Mass Number ...
Answer on Question #47967 - Chemistry – Other
... 14. amu (Atomic Mass Unit) 15. a. Number of protons 16. d. One-twelfth the mass of one carbon atom 17. Isotopes of the same element have different number of neutrons. ...
... 14. amu (Atomic Mass Unit) 15. a. Number of protons 16. d. One-twelfth the mass of one carbon atom 17. Isotopes of the same element have different number of neutrons. ...
Atoms, Ions and Molecules
... The number of neutrons does not change which element an atom belongs to. Atoms of the same element that have different numbers of neutrons are known as isotopes. Isotopes have idenBcal chemical properBes ...
... The number of neutrons does not change which element an atom belongs to. Atoms of the same element that have different numbers of neutrons are known as isotopes. Isotopes have idenBcal chemical properBes ...
2. NH3 - Huffman Chemistry Website!
... (Example: Noble gases – Elements with the outermost s and P sublevels are filled.) Transition metals – Inner transition metals – Representative elements – Alkali metals Alkaline earth metals Halogens Groups Periods ...
... (Example: Noble gases – Elements with the outermost s and P sublevels are filled.) Transition metals – Inner transition metals – Representative elements – Alkali metals Alkaline earth metals Halogens Groups Periods ...
Ch 02.01-03: Atoms Molecules Ions
... 2. All atoms of a given element have the same mass and other properties that distinguish them from the atoms of other elements. 3. Atoms combine in simple, whole-number ratios to form compounds. 4. Atoms of one element cannot change into atoms of another element. In a chemical reaction, atoms only c ...
... 2. All atoms of a given element have the same mass and other properties that distinguish them from the atoms of other elements. 3. Atoms combine in simple, whole-number ratios to form compounds. 4. Atoms of one element cannot change into atoms of another element. In a chemical reaction, atoms only c ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.