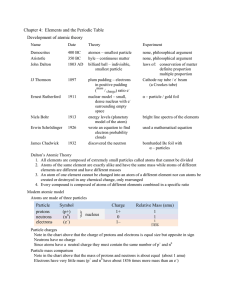
Chapter 4: Elements and the Periodic Table Development of atomic
... Mass number: the number of protons and neutrons in the nucleus of an atom Mass number defines what isotope an atom is Hyphen notation: a means of writing a single nuclide in an isotope Example: carbon–13 has 6 p+ (it is carbon) and 7 n0 (it is one of the nuclides of carbon) Calculating p+, n0, and e ...
... Mass number: the number of protons and neutrons in the nucleus of an atom Mass number defines what isotope an atom is Hyphen notation: a means of writing a single nuclide in an isotope Example: carbon–13 has 6 p+ (it is carbon) and 7 n0 (it is one of the nuclides of carbon) Calculating p+, n0, and e ...
Teaching/Chemistry/Chemistry Lesson Plans 04
... Fluorine – 3.55 x 10-23g Arsenic – 1.244 x 10-22 g o Such numbers are too small to work with o Carbon-12 was chosen as the standard and given an atomic mass of 12 (6 neutrons & 6 protons make up the mass – electrons are neglible) Carbon-12 has 12 amu 1 amu = 1/12 Carbon-12 and since there are 6 neut ...
... Fluorine – 3.55 x 10-23g Arsenic – 1.244 x 10-22 g o Such numbers are too small to work with o Carbon-12 was chosen as the standard and given an atomic mass of 12 (6 neutrons & 6 protons make up the mass – electrons are neglible) Carbon-12 has 12 amu 1 amu = 1/12 Carbon-12 and since there are 6 neut ...
Chapter 18 Notes
... *so the Atomic Number is 6 and therefore it has 6 protons, & 6 neutrons. -Isotope of carbon- Carbon-14 *Atomic # is still 6, so you have 6 protons & 8 Neutrons. *They use how much each isotope occurs and it’s mass to determine the Average atomic mass. Ex- IF you have an average atomic mass of 35.95 ...
... *so the Atomic Number is 6 and therefore it has 6 protons, & 6 neutrons. -Isotope of carbon- Carbon-14 *Atomic # is still 6, so you have 6 protons & 8 Neutrons. *They use how much each isotope occurs and it’s mass to determine the Average atomic mass. Ex- IF you have an average atomic mass of 35.95 ...
Chapter 3 The Atom
... e.g. carbon, symbol C, from the Latin word "carbo" meaning "charcoal" e.g. lead, symbol Pb, the origin of the symbol Pb is the Latin word "plumbum" e.g. mercury, symbol Hg, the origin of the symbol Hg is the Latin word "hydrargyrum" meaning "liquid silver" e.g., Bismuth, symbol Bi, from the ...
... e.g. carbon, symbol C, from the Latin word "carbo" meaning "charcoal" e.g. lead, symbol Pb, the origin of the symbol Pb is the Latin word "plumbum" e.g. mercury, symbol Hg, the origin of the symbol Hg is the Latin word "hydrargyrum" meaning "liquid silver" e.g., Bismuth, symbol Bi, from the ...
Atomic Structure
... If the number of protons changes then the identity of the element changes with it! Atomic Number (Z) – the number of protons in the nucleus of each atom of that element Z = # of protons ...
... If the number of protons changes then the identity of the element changes with it! Atomic Number (Z) – the number of protons in the nucleus of each atom of that element Z = # of protons ...
Chapter 2.1, 2.2 Review Packet – Answer Key
... Atoms of the same element that differ in the number of neutrons are called isotopes. Isotopes are identified by their mass number, the total number of protons and neutrons in the nucleus. Because they have the same number of electrons in each atom, all isotopes of an element have the same chemical p ...
... Atoms of the same element that differ in the number of neutrons are called isotopes. Isotopes are identified by their mass number, the total number of protons and neutrons in the nucleus. Because they have the same number of electrons in each atom, all isotopes of an element have the same chemical p ...
Chapter 4.3: How Atoms Differ
... When writing radioactive decomposition reactions, the mass numbers (top) and the atomic numbers (bottom) must be equal on both sides. Radioactivity Problems – write the equations for the following radioactive decomposition reactions ...
... When writing radioactive decomposition reactions, the mass numbers (top) and the atomic numbers (bottom) must be equal on both sides. Radioactivity Problems – write the equations for the following radioactive decomposition reactions ...
Isotopes
... left of the chemical symbol, For iron (Fe) we have, for example: 54Fe, 56Fe, 57Fe, and 58Fe. Since the iron has the atomic number zFe = 26, we have 54 - 26 = 28 neutrons in 54Fe, and 30, 31, and 32 neutrons, respectively, in the other three isotopes given. Isotopes come in two basic variants: 1. Rad ...
... left of the chemical symbol, For iron (Fe) we have, for example: 54Fe, 56Fe, 57Fe, and 58Fe. Since the iron has the atomic number zFe = 26, we have 54 - 26 = 28 neutrons in 54Fe, and 30, 31, and 32 neutrons, respectively, in the other three isotopes given. Isotopes come in two basic variants: 1. Rad ...
Atomic Theory and Structure Quiz
... 1. Dalton incorporated the law of conservation of mass into his atomic theory by asserting that a. matter is composed of atoms. b. atoms can be destroyed in chemical reactions. c. atoms are indivisible. 2. Oxygen can combine with carbon to form two compounds, carbon monoxide and carbon dioxide. The ...
... 1. Dalton incorporated the law of conservation of mass into his atomic theory by asserting that a. matter is composed of atoms. b. atoms can be destroyed in chemical reactions. c. atoms are indivisible. 2. Oxygen can combine with carbon to form two compounds, carbon monoxide and carbon dioxide. The ...
Elements and Compounds
... • Latin names from historical context produce unusual symbols for some elements: first letter is capitalized, second is lower case – Ferrum: Fe (Iron) – Aurum: Au (Gold) – Cobalt: Co ...
... • Latin names from historical context produce unusual symbols for some elements: first letter is capitalized, second is lower case – Ferrum: Fe (Iron) – Aurum: Au (Gold) – Cobalt: Co ...
PowerPoint Presentation - The Atom: Chp 12 sect 2
... some isotopes may be different. • Some isotopes are radioactivemeaning they "radiate" energy as they decay to a more stable form, • perhaps another element half-life: time required for half of the atoms of an element to decay into stable form. ...
... some isotopes may be different. • Some isotopes are radioactivemeaning they "radiate" energy as they decay to a more stable form, • perhaps another element half-life: time required for half of the atoms of an element to decay into stable form. ...
Atoms
... Atoms An atom’s atomic number has many uses. It tells you where to find it on the periodic table, obviously (hydrogen is 1 and helium is 2), but it also tells you the number of protons found in the atom’s nucleus. If an atom is neutral (meaning it has a charge of 0), then its number of protons (posi ...
... Atoms An atom’s atomic number has many uses. It tells you where to find it on the periodic table, obviously (hydrogen is 1 and helium is 2), but it also tells you the number of protons found in the atom’s nucleus. If an atom is neutral (meaning it has a charge of 0), then its number of protons (posi ...
Ch 3: Atoms
... nucleus - also indicates the # of electrons if the element is not charged atomic mass – the average mass of all of the isotopes of an element – is a number with a decimal – is always the larger number on the periodic table. mass number (A) - sum of the protons and neutrons in a nucleus this number i ...
... nucleus - also indicates the # of electrons if the element is not charged atomic mass – the average mass of all of the isotopes of an element – is a number with a decimal – is always the larger number on the periodic table. mass number (A) - sum of the protons and neutrons in a nucleus this number i ...
Name January 5, 2017 Period Bio-Chem Unit 2 Review (Chapters
... Aristotle: Matter is made of Air, Fire, Earth, & Water. Democritus: matter is not continuous but is composed of tiny, discrete, indivisible particles called “atomos,” (atoms). C. 1808 John Dalton was first to publish Atomic Theory a. All matter composed of indivisible particles called atoms, which r ...
... Aristotle: Matter is made of Air, Fire, Earth, & Water. Democritus: matter is not continuous but is composed of tiny, discrete, indivisible particles called “atomos,” (atoms). C. 1808 John Dalton was first to publish Atomic Theory a. All matter composed of indivisible particles called atoms, which r ...
File
... • Sometimes an element has a number of atoms that have differing numbers of neutrons • Atoms with the same number of protons but different numbers of neutrons are called isotopes. • True or false: • Two isotopes of the same element will have a different atomic number. • Two isotopes of the same elem ...
... • Sometimes an element has a number of atoms that have differing numbers of neutrons • Atoms with the same number of protons but different numbers of neutrons are called isotopes. • True or false: • Two isotopes of the same element will have a different atomic number. • Two isotopes of the same elem ...
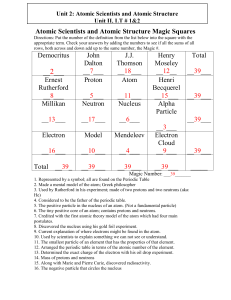
Atomic Scientists and Atomic Structure Magic Squares
... 5. The positive particle in the nucleus of an atom. (Not a fundamental particle) 6. The tiny positive core of an atom; contains protons and neutrons. 7. Credited with the first atomic theory model of the atom which had four main postulates. 8. Discovered the nucleus using his gold foil experiment. 9 ...
... 5. The positive particle in the nucleus of an atom. (Not a fundamental particle) 6. The tiny positive core of an atom; contains protons and neutrons. 7. Credited with the first atomic theory model of the atom which had four main postulates. 8. Discovered the nucleus using his gold foil experiment. 9 ...
Unit 3 Test - hrsbstaff.ednet.ns.ca
... b) a change in state from gas to solid, or from solid to gas ...
... b) a change in state from gas to solid, or from solid to gas ...
atoms - Net Start Class
... are composed of extremely small irreducible particles called atoms. • The atomic theory was roundly rejected by Aristotle, and, thus, by almost everybody else for the next two millennia. ...
... are composed of extremely small irreducible particles called atoms. • The atomic theory was roundly rejected by Aristotle, and, thus, by almost everybody else for the next two millennia. ...
Chemistry II Chapter 2 Notes
... • He also stated that atoms of one element are all the same and different from those of other elements • Atoms combine in small whole number ratios to form compounds. • In chemical reactions atoms are just rearranged. ...
... • He also stated that atoms of one element are all the same and different from those of other elements • Atoms combine in small whole number ratios to form compounds. • In chemical reactions atoms are just rearranged. ...
cell molecules
... Matter consists of chemical elements in pure form and in combinations called compounds • Organisms are composed of matter. • Matter is anything that takes up space and has mass. • An element is a substance that cannot be broken down to other substances by chemical reactions. • There are 92 naturall ...
... Matter consists of chemical elements in pure form and in combinations called compounds • Organisms are composed of matter. • Matter is anything that takes up space and has mass. • An element is a substance that cannot be broken down to other substances by chemical reactions. • There are 92 naturall ...
Writing Formulas
... Remember the algebraic sum of the ions' oxidation numbers must equal zero. (Balance) Learn the polyatomic ions. Learn those ions with multiple oxidation numbers and use Roman numerals to indicate the charge. ...
... Remember the algebraic sum of the ions' oxidation numbers must equal zero. (Balance) Learn the polyatomic ions. Learn those ions with multiple oxidation numbers and use Roman numerals to indicate the charge. ...
Chapter 14 ~ Atoms
... seven electrons in their outer energy levels. One similar property of the halogens is the ability to form compounds with elements in Group 1. The elements in Group 18 are known as noble gases. Noble gases do not usually form compounds. We say they are stable, or unreactive. The atoms of all the nobl ...
... seven electrons in their outer energy levels. One similar property of the halogens is the ability to form compounds with elements in Group 1. The elements in Group 18 are known as noble gases. Noble gases do not usually form compounds. We say they are stable, or unreactive. The atoms of all the nobl ...
Chapter 2: Atoms Molecules and Ions
... reactant molecules into a mass ratio for a chemical reaction to be useful. 2) Mass ratios are determined by using atomic masses for the elements. i) Atomic masses (atomic weights) are found in periodic table beneath the chemical symbol, and represent the average of all the naturally occurring isotop ...
... reactant molecules into a mass ratio for a chemical reaction to be useful. 2) Mass ratios are determined by using atomic masses for the elements. i) Atomic masses (atomic weights) are found in periodic table beneath the chemical symbol, and represent the average of all the naturally occurring isotop ...
Atomic Masses
... Dalton’s atomic theory. Identify the parts of an atom, their location, charge, and relative mass. Determine the numbers of subatomic particles in an atom. ...
... Dalton’s atomic theory. Identify the parts of an atom, their location, charge, and relative mass. Determine the numbers of subatomic particles in an atom. ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.