File - Jolyon Johnson
... • 3 NADH, 1 FADH2, 1 ATP produced per cycle • Fats are “stored energy” because they break down into acetate and enter the Krebs cycle • Ketoglutarate, succinate, fumarate, and malate form into amino acids to build proteins • There are two cycles for one glucose molecule ...
... • 3 NADH, 1 FADH2, 1 ATP produced per cycle • Fats are “stored energy” because they break down into acetate and enter the Krebs cycle • Ketoglutarate, succinate, fumarate, and malate form into amino acids to build proteins • There are two cycles for one glucose molecule ...
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-ISSN: 2278-3008, p-ISSN:2319-7676.
... Mitochondria enclose the biochemical machinery for cellular respiration; the aerobic processes by which sugars, fatty acids, and amino acids are broken down into carbon dioxide & water and their chemical energy is entrapped as ATP. The Kreb’s cycle, also called tri-carboxylic acid or citric acid cyc ...
... Mitochondria enclose the biochemical machinery for cellular respiration; the aerobic processes by which sugars, fatty acids, and amino acids are broken down into carbon dioxide & water and their chemical energy is entrapped as ATP. The Kreb’s cycle, also called tri-carboxylic acid or citric acid cyc ...
Alcoholic fermentation
... …………………….. back to NAD+ so that the energy yielding phase of glycolysis can continue. In yeast, pyruvate is decarboxylated to ETHANAL (…..C), releasing …………….. . The enzyme alcohol dehydrogenase then ……………….. ETHANAL to ETHANOL (…..C), at the same time ………………… NADH back to ……………. . CH3CHO + NADH ...
... …………………….. back to NAD+ so that the energy yielding phase of glycolysis can continue. In yeast, pyruvate is decarboxylated to ETHANAL (…..C), releasing …………….. . The enzyme alcohol dehydrogenase then ……………….. ETHANAL to ETHANOL (…..C), at the same time ………………… NADH back to ……………. . CH3CHO + NADH ...
Chapter Outline
... a. The ETC consists of three protein complexes and two carriers that transport electrons. b. The three protein complexes include NADH-Q reductase complex, the cytochrome reductase complex, and the cytochrome oxidase complex; the two protein mobile carriers are coenzyme Q and cytochrome c. c. Energy ...
... a. The ETC consists of three protein complexes and two carriers that transport electrons. b. The three protein complexes include NADH-Q reductase complex, the cytochrome reductase complex, and the cytochrome oxidase complex; the two protein mobile carriers are coenzyme Q and cytochrome c. c. Energy ...
Model 2 – Amylase Rate of Reaction
... channel for the hydrogen ions to pass through the membrane? 37. The flow of hydrogen ions through the protein channel provides free energy to do work. What process (circled) in Model 2 requires energy? ...
... channel for the hydrogen ions to pass through the membrane? 37. The flow of hydrogen ions through the protein channel provides free energy to do work. What process (circled) in Model 2 requires energy? ...
Latest research findings Developing the Day
... • 2,1 billion years ago, end of first total glaciation period on Earth, for the first time oxygen appeared in the atmosphere, methane decreased • Fusion of • Methanogenic Archaea (look like bacteria, but are not), live in extreme environments (still today in the deep sea), never meet light, produce ...
... • 2,1 billion years ago, end of first total glaciation period on Earth, for the first time oxygen appeared in the atmosphere, methane decreased • Fusion of • Methanogenic Archaea (look like bacteria, but are not), live in extreme environments (still today in the deep sea), never meet light, produce ...
Chapter 9 outline
... In the electron transport chain – Electrons from NADH and FADH2 lose energy in several steps ATP synthase – Is the enzyme that actually makes ATP At certain steps along the electron transport chain – Electron transfer causes protein complexes to pump H+ from the mitochondrial matrix to the intermemb ...
... In the electron transport chain – Electrons from NADH and FADH2 lose energy in several steps ATP synthase – Is the enzyme that actually makes ATP At certain steps along the electron transport chain – Electron transfer causes protein complexes to pump H+ from the mitochondrial matrix to the intermemb ...
Answers - U of L Class Index
... The NADH produced from glycolysis cannot pass through the mitochondrial membrane. Therefore, the hydrogen ions and electrons from the NADH in the cytoplasm are transferred to dihydroxyacetone phosphate, which is reduced to glycerol-3-phosphate to regenerate NAD+. After glycerol-3-phosphate moves int ...
... The NADH produced from glycolysis cannot pass through the mitochondrial membrane. Therefore, the hydrogen ions and electrons from the NADH in the cytoplasm are transferred to dihydroxyacetone phosphate, which is reduced to glycerol-3-phosphate to regenerate NAD+. After glycerol-3-phosphate moves int ...
Document
... increase in [lactate] so that the [lactate]/[pyruvate] ratio is many times larger than normal. Explain. ...
... increase in [lactate] so that the [lactate]/[pyruvate] ratio is many times larger than normal. Explain. ...
Chapter 4: Energy and Cellular Metabolism, Part 2
... Energy transferred commonly measured in calories: 1 cal = 1 g of H2O by 1° C 1 Kcal = temp. of 1L H2O by 1o C. = Calorie (capital C) ...
... Energy transferred commonly measured in calories: 1 cal = 1 g of H2O by 1° C 1 Kcal = temp. of 1L H2O by 1o C. = Calorie (capital C) ...
ch3b FA11 - Cal State LA
... Oxidation and reduction • Redox reactions: the gain (reduction) or loss (oxidation) of electrons – Changes in organic molecules shift the degree of e- sharing • Carbon in C-H bond is reduced • Carbon in C=O bond is oxidized – EN diffs result in e- spending less time around C when bonded to O ...
... Oxidation and reduction • Redox reactions: the gain (reduction) or loss (oxidation) of electrons – Changes in organic molecules shift the degree of e- sharing • Carbon in C-H bond is reduced • Carbon in C=O bond is oxidized – EN diffs result in e- spending less time around C when bonded to O ...
Lecture 11: Take your Vitamins! Enzyme Cofactors Reference
... 4. For each cofactor example given in the slides, name the functional part of the molecule (the “business end”) and the type of reaction it participates in (also named in Table 7-1). A. Metabolite Cofactors: Molecules that are produced by metabolic pathways that are used by other enzymes to carry o ...
... 4. For each cofactor example given in the slides, name the functional part of the molecule (the “business end”) and the type of reaction it participates in (also named in Table 7-1). A. Metabolite Cofactors: Molecules that are produced by metabolic pathways that are used by other enzymes to carry o ...
Aerobic Metabolism: The Citric Acid Cycle
... In aerobic organisms, the citric acid cycle is part of a metabolic pathway involved in the chemical conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy. The citric acid cycle also provides precursors for many compounds such as certain amin ...
... In aerobic organisms, the citric acid cycle is part of a metabolic pathway involved in the chemical conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy. The citric acid cycle also provides precursors for many compounds such as certain amin ...
Aerobic Metabolism: The Citric Acid Cycle
... In aerobic organisms, the citric acid cycle is part of a metabolic pathway involved in the chemical conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy. The citric acid cycle also provides precursors for many compounds such as certain amin ...
... In aerobic organisms, the citric acid cycle is part of a metabolic pathway involved in the chemical conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy. The citric acid cycle also provides precursors for many compounds such as certain amin ...
Q01to05
... ATP = 4.8, ADP = 0.2, AMP in uM The total adenine nucleotide pool ([ATP] + [ADP] + [AMP]) in cells is about 5 mM ATP = 4.8, ADP = 0.2, AMP in uM ...
... ATP = 4.8, ADP = 0.2, AMP in uM The total adenine nucleotide pool ([ATP] + [ADP] + [AMP]) in cells is about 5 mM ATP = 4.8, ADP = 0.2, AMP in uM ...
Mathematics Semester 1 Study Guide
... 8. What are polymers and how are they made? 9. What is a condensation or dehydration synthesis reaction? 10. What is a hydrolysis reaction? How is water involved in this type of reaction? 11. What are the four major classes of organic compounds? 12. What organic compound class includes the sugars an ...
... 8. What are polymers and how are they made? 9. What is a condensation or dehydration synthesis reaction? 10. What is a hydrolysis reaction? How is water involved in this type of reaction? 11. What are the four major classes of organic compounds? 12. What organic compound class includes the sugars an ...
The Citric Acid Cycle - Rubin Risto Gulaboski
... • The reactions of metabolism are MANY • In this class we will discuss some of the major reactions: – Glyco - Lysis (glycolysis) – The Citric Acid Cycle – The Electron Transport Chain ...
... • The reactions of metabolism are MANY • In this class we will discuss some of the major reactions: – Glyco - Lysis (glycolysis) – The Citric Acid Cycle – The Electron Transport Chain ...
Cell Respiration Key
... matrix, CO2, NADH, Krebs Cycle, Glycolysis, Cytoplasm, ATP, Glucose, inner membrane and FADH2. ...
... matrix, CO2, NADH, Krebs Cycle, Glycolysis, Cytoplasm, ATP, Glucose, inner membrane and FADH2. ...
Column A
... a. What are the atomic mass units for protons, neutrons, and electrons? Protons and neutrons = 1 amu; electrons about 0 amu What does the atomic number represent? # of protons b. What does the mass number represent? # of protons + # of neutrons c. What particles are in equal numbers in a neutral ato ...
... a. What are the atomic mass units for protons, neutrons, and electrons? Protons and neutrons = 1 amu; electrons about 0 amu What does the atomic number represent? # of protons b. What does the mass number represent? # of protons + # of neutrons c. What particles are in equal numbers in a neutral ato ...
anaerobic respiration
... • coupled to the reduction step is an oxidation involving Fe or Cu: Fe2+ Fe3+ + e or Cu+ Cu2+ + e ...
... • coupled to the reduction step is an oxidation involving Fe or Cu: Fe2+ Fe3+ + e or Cu+ Cu2+ + e ...
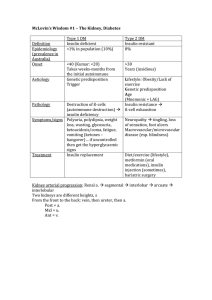
McLovin`s Wisdom #1 – The Kidney, Diabetes Type 1 DM Type 2
... 10 H+ inside from NADH and 6+ inside from FADH2. Each ATP needs a total of H+ to be produced. Electron transport chain ...
... 10 H+ inside from NADH and 6+ inside from FADH2. Each ATP needs a total of H+ to be produced. Electron transport chain ...
cellular respiration
... forming NADH. – Uses two ATP molecules per glucose to split the six-carbon glucose – Makes four additional ATP directly when enzymes transfer phosphate groups from fuel molecules to ADP ...
... forming NADH. – Uses two ATP molecules per glucose to split the six-carbon glucose – Makes four additional ATP directly when enzymes transfer phosphate groups from fuel molecules to ADP ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.